In vivo roles of the basic domain of dynactin p150 in microtubule plus-end tracking and dynein function - PubMed (original) (raw)
In vivo roles of the basic domain of dynactin p150 in microtubule plus-end tracking and dynein function
Xuanli Yao et al. Traffic. 2012 Mar.
Abstract
Microtubule (MT) plus-end-tracking proteins accumulate at MT plus ends for various cellular functions, but their targeting mechanisms are not fully understood (Akhmanova A and Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol 2008;9:309-322.). Here, we tested in the filamentous fungus Aspergillus nidulans the requirement for plus-end localization of dynactin p150, a protein essential for dynein function. Deletion of the N-terminal MT-binding region of p150 significantly diminishes the MT plus-end accumulation of both dynein heavy chain and p150, and causes a partial defect in nuclear distribution. Surprisingly, within the MT-binding region, the basic domain is more critical than the CAP-Gly (cytoskeleton-associated protein glycine-rich) domain for maintaining plus-end tracking of p150, as well as for the functions of dynein in nuclear distribution and early endosome movement. Our results show that the basic domain of A. nidulans p150 is important for p150-MT interaction both in vivo and in vitro, and the basic amino acids within this domain are crucial for the plus-end accumulation of p150 in the wild-type background and for the p150-MT interaction in the ΔkinA (kinesin-1) background. We suggest that the basic amino acids are required for the electrostatic interaction between p150 and MTs, which is important for kinesin-1-mediated plus-end targeting of dynactin and dynein in A. nidulans.
© 2011 John Wiley & Sons A/S.
Figures
Figure 1
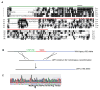
A sequence alignment of the N-terminal p150 proteins from different species (A), strategy for creating the ΔMT-p150 allele in A. nidulans (B) and sequencing analysis of the ΔMT-p150 allele (C). (A) The N-terminal 200 amino acids of p150 proteins from different species were aligned and identical amino acids were boxed in black. A.n.: Aspergillus nidulans; N.c.: Neurospora crassa; S.c.: Saccharomyces cerevisiae; D.m.: Drosophila melanogaster; H.s.: Homo sapiens. Green line indicates the CAP-Gly domain (aa 3-64) and red line indicates the basic domain (aa 65-161) of A. nidulans p150. The end of the CAP-Gly domain and the beginning of the basic domain are defined according to Figure 1C of Culver-Hanlon et al., 2006 (17). Asterisks indicate the highly conserved GKNDG residues within the CAP-Gly domain. (B) Construction of the A. nidulans ΔMT-p150 allele using homologous recombination. (C) Sequence of the ΔMT-p150 allele. Sequencing analysis was done using genomic DNA isolated from the ΔMT mutant. An amino acid sequence is shown below the nucleotide sequence, and arrow indicates the position of deletion.
Figure 2
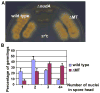
Colony and nuclear-distribution phenotype of the ΔMT mutant. (A) Colony morphology of strains containing the wild-type p150 allele, the ΔMT-p150 allele, or the Δ_nudA_ (dynein HC) allele. Strains were grown on a Y+UU plate for 2 days at 37°C. Note that the ΔMT mutant is much healthier than the Δ_nudA_ mutant. However, the color of the ΔMT mutant colony appears slightly dimmer than that of wild type. (B) A quantitative analysis of nuclear distribution in the ΔMT mutant in comparison to that in a wild type control strain. Strains were grown in liquid Y+UU medium for 7 hours at 37°C before being fixed and stained with DAPI for visualizing the nuclei. Note that in the ΔMT mutant, the percentage of cells containing one or two nuclei in the spore head is significantly lower than that of wild type (p<0.01), while the percentage of cells containing three, four or more nuclei in the spore head is significantly higher than that of wild type (p<0.01). Means and standard deviations were calculated from three independent experiments, and for each experiment, more than 150 germlings were analyzed.
Figure 3
The MT-binding region of p150 is not important for dynein-dynactin interaction but important for the microtubule plus-end accumulation of dynein and dynactin. (A) S-tagged IC pulls down similar amounts of p150 and ΔMT-p150. Western blots were probed with antibodies against dynein IC and dynactin p150. (B) The plus-end comets formed by GFP-HC in wild type are not seen in the ΔMT-p150 mutant. (C) While p150-GFP forms plus-end comets, ΔMT-p150-GFP does not form plus-end comets. Bar, 5 μm.
Figure 4
Construction of the ΔCAP-Gly and Δbasic mutants and analyses of their colony and nuclear-distribution phenotypes. (A) A diagram showing the wild-type p150 protein verses the ΔMT, ΔCAP-Gly and Δbasic mutant p150 proteins. CC1 (coiled-coil domain 1, aa 218-556) and CC2 (aa 936-1098) as identified by the SMART program are also indicated. (B) Results of sequencing the genomic DNAs of the ΔCAP-Gly and Δbasic mutants. Since a reverse primer was used, the sequences represent that of the reverse complementing stand of the coding strand. Positions of deletion are indicated by arrows. (C) Colony morphology of wild type, the ΔCAP-Gly and the Δbasic mutants at 37°C and 42°C. (D) An analysis of nuclear distribution in the ΔCAP-Gly and Δbasic mutants. The analysis was done similarly to what has been described for the ΔMT mutant in Figure 2. Note that in the Δbasic mutant, the percentage of cells containing one or two nuclei in the spore head is significantly lower than that in wild type (p<0.01), while the percentage of cells containing three, four or more nuclei in the spore head is significantly higher than that in wild type (p<0.01). The nuclear distribution pattern of the ΔCAP-Gly mutant is similar to that of wild type except for a mild but statistically significant increase in the percentage of cells containing four or more nuclei in the spore head (p<0.01).
Figure 5
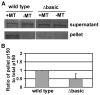
The basic domain of A. nidulans p150 is important for p150-MT interaction in vitro. (A) Results of a microtubule (MT)-pelleting assay shown by a western blot probed with an anti-GFP antibody to reveal the level of p150-GFP (wild type) and that of Δbasic-p150-GFP (Δbasic). Samples with no microtubules added (−MT) were used as negative controls. (B) In the presence of microtubules (+MT), the ratio of pellet p150 to total p150 (pellet + supernatant) is significantly lower in the Δbasic mutant than in wild type (p<0.05). Values are relative to the wild type value, which is set at 1. Mean and standard deviation values were calculated from five independent MT-pelleting experiments.
Figure 6
The basic domain of A. nidulans p150 is important for the microtubule plus-end accumulation of dynactin and dynein in vivo. (A) While p150-GFP and ΔCAP-Gly-p150-GFP form the plus-end comets, Δbasic-p150-GFP does not form the comets, and GFP-HC does not form plus-end comets either in the Δbasic mutant. (B) GFP-CLIPA forms plus-end comets in both wild type and the Δbasic p150 mutant, indicating no gross alteration in the microtubule plus end structure in the Δbasic mutant. (C) Western analyses of total protein extract using an anti-GFP antibody and an anti-dynein-IC antibody indicate that the protein levels of p150-GFP (wild type), ΔMT-p150-GFP (ΔMT), ΔCAP-Gly-p150-GFP (ΔCAP-Gly) and Δbasic-p150-GFP (Δbasic) are similar. Bar, 5 μm.
Figure 7
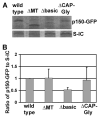
Removing the basic domain of p150 affects dynein-dynactin interaction. (A) Western blots showing that the S-tagged IC (S-IC) pulls down p150-GFP (wild type), ΔMT-p150-GFP (ΔMT), Δbasic-p150-GFP (Δbasic) and ΔCAP-Gly-p150-GFP (ΔCAP-Gly) proteins from respective strains. (B) A quantitative analysis of the pull-down results showing that the amount of pulled-down Δbasic-p150-GFP is lower than that of wild type p150-GFP (p<0.05), while that of pulled-down ΔMT-p150-GFP or ΔCAP-Gly-p150-GFP is not significantly lower than that of wild type at a p value of 0.05. Values are relative to the wild type value, which is set at 1. Mean and standard deviation values were calculated from three independent experiments.
Figure 8
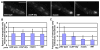
The Δbasic mutant exhibits a defect in dynein-mediated retrograde early endosome transport away from the hyphal tip. (A) Early endosomes labeled with mCherry-RabA distribute along the hyphae in wild type and ΔCAP-Gly mutant but exhibit an abnormal hyphal-tip accumulation in the Δbasic and ΔMT mutants. (B) Speed of retrograde early endosome transport away from the hyphal tip. The speed in the ΔCAP-Gly, Δbasic or ΔMT mutant is not significantly different from that of wild type at a p value of 0.05. Mean and S.D. are shown (n=15 for wild type, n=11 for ΔCAP-Gly, n=10 for Δbasic and n=16 for ΔMT). (C) Frequency of retrograde early endosome movement. This is defined as the number of mCherry-RabA-positive particles moving away from the hyphal tip in a single hypha within a 16-sec period. The frequency in the ΔCAP-Gly mutant is not significantly lower than that of wild type at a p value of 0.05, but that in the Δbasic or the ΔMT mutant is significantly lower than that of wild type (p<0.05). Mean and standard deviation values are shown (n=14 hyphae for all strains). Bar, 5 μm.
Figure 9
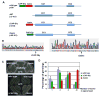
The Δbasic and Ala-basic mutant p150 proteins are defective in interacting with microtubules in vivo. (A) In the Δ_kinA_ background, p150-GFP and ΔCAP-Gly-p150-GFP decorate MT-like structures while Δbasic-p150-GFP only exhibits background fluorescence. (B) Ala-basic-p150-GFP fails to form bright plus-end comets as those formed by p150-GFP, and Ala-basic-p150-GFP also fails to decorates MT-like structures in the Δ_kinA_ background. Bar, 5 μm. (C) Western blots showing that the S-tagged IC (S-IC) pulls down p150-GFP (wild type) and Ala-basic-p150-GFP (Ala-basic) proteins from respective strains. (D) A quantitative analysis of the pull-down results showing that the amount of pulled-down Ala-basic-p150-GFP is not significantly different from that of wild type p150-GFP at a p value of 0.05. Values are relative to the wild type value, which is set at 1. Mean and standard deviation values were calculated from four independent experiments.
Similar articles
- Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent.
Zhang J, Li S, Fischer R, Xiang X. Zhang J, et al. Mol Biol Cell. 2003 Apr;14(4):1479-88. doi: 10.1091/mbc.e02-08-0516. Mol Biol Cell. 2003. PMID: 12686603 Free PMC article. - p25 of the dynactin complex plays a dual role in cargo binding and dynactin regulation.
Qiu R, Zhang J, Xiang X. Qiu R, et al. J Biol Chem. 2018 Oct 5;293(40):15606-15619. doi: 10.1074/jbc.RA118.004000. Epub 2018 Aug 24. J Biol Chem. 2018. PMID: 30143531 Free PMC article. - Arp11 affects dynein-dynactin interaction and is essential for dynein function in Aspergillus nidulans.
Zhang J, Wang L, Zhuang L, Huo L, Musa S, Li S, Xiang X. Zhang J, et al. Traffic. 2008 Jul;9(7):1073-87. doi: 10.1111/j.1600-0854.2008.00748.x. Epub 2008 Apr 11. Traffic. 2008. PMID: 18410488 Free PMC article. - Cytoplasmic dynein and early endosome transport.
Xiang X, Qiu R, Yao X, Arst HN Jr, Peñalva MA, Zhang J. Xiang X, et al. Cell Mol Life Sci. 2015 Sep;72(17):3267-80. doi: 10.1007/s00018-015-1926-y. Epub 2015 May 23. Cell Mol Life Sci. 2015. PMID: 26001903 Free PMC article. Review. - Microtubule plus ends, motors, and traffic of Golgi membranes.
Vaughan KT. Vaughan KT. Biochim Biophys Acta. 2005 Jul 10;1744(3):316-24. doi: 10.1016/j.bbamcr.2005.05.001. Biochim Biophys Acta. 2005. PMID: 15950296 Review.
Cited by
- Identification of a novel site in the tail of dynein heavy chain important for dynein function in vivo.
Qiu R, Zhang J, Xiang X. Qiu R, et al. J Biol Chem. 2013 Jan 25;288(4):2271-80. doi: 10.1074/jbc.M112.412403. Epub 2012 Dec 3. J Biol Chem. 2013. PMID: 23212922 Free PMC article. - Cargo-Mediated Activation of Cytoplasmic Dynein in vivo.
Xiang X, Qiu R. Xiang X, et al. Front Cell Dev Biol. 2020 Oct 23;8:598952. doi: 10.3389/fcell.2020.598952. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195284 Free PMC article. Review. - Microtubule-based transport in filamentous fungi.
Egan MJ, McClintock MA, Reck-Peterson SL. Egan MJ, et al. Curr Opin Microbiol. 2012 Dec;15(6):637-45. doi: 10.1016/j.mib.2012.10.003. Epub 2012 Nov 2. Curr Opin Microbiol. 2012. PMID: 23127389 Free PMC article. Review. - Transportation of Aspergillus nidulans Class III and V Chitin Synthases to the Hyphal Tips Depends on Conventional Kinesin.
Takeshita N, Wernet V, Tsuizaki M, Grün N, Hoshi HO, Ohta A, Fischer R, Horiuchi H. Takeshita N, et al. PLoS One. 2015 May 8;10(5):e0125937. doi: 10.1371/journal.pone.0125937. eCollection 2015. PLoS One. 2015. PMID: 25955346 Free PMC article. - The actin capping protein in Aspergillus nidulans enhances dynein function without significantly affecting Arp1 filament assembly.
Zhang J, Qiu R, Xiang X. Zhang J, et al. Sci Rep. 2018 Jul 30;8(1):11419. doi: 10.1038/s41598-018-29818-4. Sci Rep. 2018. PMID: 30061726 Free PMC article.
References
- Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. - PubMed
- Pearson CG, Bloom K. Dynamic microtubules lead the way for spindle positioning. Nat Rev Mol Cell Biol. 2004;5:481–492. - PubMed
- Carvalho P, Tirnauer JS, Pellman D. Surfing on microtubule ends. Trends Cell Biol. 2003;13:229–237. - PubMed
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. - PubMed
- Wu X, Xiang X, Hammer JA., 3rd Motor proteins at the microtubule plus-end. Trends Cell Biol. 2006;16:135–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous