PET amyloid-beta imaging in preclinical Alzheimer's disease - PubMed (original) (raw)
Review
PET amyloid-beta imaging in preclinical Alzheimer's disease
Andrei G Vlassenko et al. Biochim Biophys Acta. 2012 Mar.
Abstract
Alzheimer's disease (AD) is the leading cause of dementia, accounting for 60-70% of all cases [Hebert et al., 2003, 1]. The need for effective therapies for AD is great. Current approaches, including cholinesterase inhibitors and N-methyl-d-aspartate (NMDA) receptor antagonists, are symptomatic treatments for AD but do not prevent disease progression. Many diagnostic and therapeutic approaches to AD are currently changing due to the knowledge that underlying pathology starts 10 to 20 years before clinical signs of dementia appear [Holtzman et al., 2011, 2]. New therapies which focus on prevention or delay of the onset or cognitive symptoms are needed. Recent advances in the identification of AD biomarkers now make it possible to detect AD pathology in the preclinical stage of the disease, in cognitively normal (CN) individuals; this biomarker data should be used in the selection of high-risk populations for clinical trials. In vivo visualization of AD neuropathology and biological, biochemical or physiological confirmation of the effects of treatment likely will substantially improve development of novel pharmaceuticals. Positron emission tomography (PET) is the leading neuroimaging tool to detect and provide quantitative measures of AD amyloid pathology in vivo at the early stages and follow its course longitudinally. This article is part of a Special Issue entitled: Imaging Brain Aging and Neurodegenerative disease.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
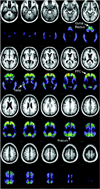
Figure 1
Mean MRI and PET [11C]PIB distribution in a standard atlas coordinate system from 10 subjects with dementia of the Alzheimer type. PET data represent [11C]PIB activity in the late (30 to 60 minutes after injection) distribution and has been normalized to standardize display and increase contrast. Brain areas used to detect raised [11C] PIB uptake in nondemented subjects are indicated with arrows. PFC = prefrontal cortex; Temp = temporal cortex; Precun = precuneus region. Reproduced with permission from [84].
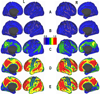
Figure 2
Schematic maps showing [11C] PIB BP distribution on lateral and medial cortical surfaces of the left (L) and right (R) hemispheres of the human brain. A, Healthy young (< 50 y. o) adults; **B–D**, Cognitively normal older (> 50 y. o.) adults with low (B), moderate (C) and high (D) Aβ deposition; E, Individuals with dementia of Alzheimer’s type (modified from Vlassenko et al. 2010 with permission).
Similar articles
- In vivo detection of microstructural correlates of brain pathology in preclinical and early Alzheimer Disease with magnetic resonance imaging.
Zhao Y, Raichle ME, Wen J, Benzinger TL, Fagan AM, Hassenstab J, Vlassenko AG, Luo J, Cairns NJ, Christensen JJ, Morris JC, Yablonskiy DA. Zhao Y, et al. Neuroimage. 2017 Mar 1;148:296-304. doi: 10.1016/j.neuroimage.2016.12.026. Epub 2016 Dec 15. Neuroimage. 2017. PMID: 27989773 Free PMC article. - Patterns of Cortical and Subcortical Amyloid Burden across Stages of Preclinical Alzheimer's Disease.
Edmonds EC, Bangen KJ, Delano-Wood L, Nation DA, Furst AJ, Salmon DP, Bondi MW; Alzheimer’s Disease Neuroimaging Initiative. Edmonds EC, et al. J Int Neuropsychol Soc. 2016 Nov;22(10):978-990. doi: 10.1017/S1355617716000928. J Int Neuropsychol Soc. 2016. PMID: 27903335 Free PMC article. - Fluid and PET biomarkers for amyloid pathology in Alzheimer's disease.
Cohen AD, Landau SM, Snitz BE, Klunk WE, Blennow K, Zetterberg H. Cohen AD, et al. Mol Cell Neurosci. 2019 Jun;97:3-17. doi: 10.1016/j.mcn.2018.12.004. Epub 2018 Dec 8. Mol Cell Neurosci. 2019. PMID: 30537535 Review. - Two-year follow-up of amyloid deposition in patients with Alzheimer's disease.
Engler H, Forsberg A, Almkvist O, Blomquist G, Larsson E, Savitcheva I, Wall A, Ringheim A, Långström B, Nordberg A. Engler H, et al. Brain. 2006 Nov;129(Pt 11):2856-66. doi: 10.1093/brain/awl178. Epub 2006 Jul 19. Brain. 2006. PMID: 16854944 - Secondary prevention of Alzheimer's dementia: neuroimaging contributions.
Ten Kate M, Ingala S, Schwarz AJ, Fox NC, Chételat G, van Berckel BNM, Ewers M, Foley C, Gispert JD, Hill D, Irizarry MC, Lammertsma AA, Molinuevo JL, Ritchie C, Scheltens P, Schmidt ME, Visser PJ, Waldman A, Wardlaw J, Haller S, Barkhof F. Ten Kate M, et al. Alzheimers Res Ther. 2018 Oct 30;10(1):112. doi: 10.1186/s13195-018-0438-z. Alzheimers Res Ther. 2018. PMID: 30376881 Free PMC article. Review.
Cited by
- Amyloid Structural Changes Studied by Infrared Microspectroscopy in Bigenic Cellular Models of Alzheimer's Disease.
Paulus A, Engdahl A, Yang Y, Boza-Serrano A, Bachiller S, Torres-Garcia L, Svanbergsson A, Garcia MG, Gouras GK, Li JY, Deierborg T, Klementieva O. Paulus A, et al. Int J Mol Sci. 2021 Mar 26;22(7):3430. doi: 10.3390/ijms22073430. Int J Mol Sci. 2021. PMID: 33810433 Free PMC article. - Linking the Amyloid, Tau, and Mitochondrial Hypotheses of Alzheimer's Disease and Identifying Promising Drug Targets.
Fišar Z. Fišar Z. Biomolecules. 2022 Nov 11;12(11):1676. doi: 10.3390/biom12111676. Biomolecules. 2022. PMID: 36421690 Free PMC article. Review. - Amyloid pathology fingerprint differentiates post-traumatic stress disorder and traumatic brain injury.
Mohamed AZ, Cumming P, Srour H, Gunasena T, Uchida A, Haller CN, Nasrallah F; Department of Defense Alzheimer's Disease Neuroimaging Initiative. Mohamed AZ, et al. Neuroimage Clin. 2018 Jun 5;19:716-726. doi: 10.1016/j.nicl.2018.05.016. eCollection 2018. Neuroimage Clin. 2018. PMID: 30009128 Free PMC article. - Human non-REM sleep and the mean global BOLD signal.
McAvoy MP, Tagliazucchi E, Laufs H, Raichle ME. McAvoy MP, et al. J Cereb Blood Flow Metab. 2019 Nov;39(11):2210-2222. doi: 10.1177/0271678X18791070. Epub 2018 Aug 3. J Cereb Blood Flow Metab. 2019. PMID: 30073858 Free PMC article. - Altered Energy Metabolism Pathways in the Posterior Cingulate in Young Adult Apolipoprotein E ɛ4 Carriers.
Perkins M, Wolf AB, Chavira B, Shonebarger D, Meckel JP, Leung L, Ballina L, Ly S, Saini A, Jones TB, Vallejo J, Jentarra G, Valla J. Perkins M, et al. J Alzheimers Dis. 2016 Apr 23;53(1):95-106. doi: 10.3233/JAD-151205. J Alzheimers Dis. 2016. PMID: 27128370 Free PMC article.
References
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. - PubMed
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann.Neurol. 1999;45:358–368. - PubMed
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–1402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG003991-29/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P50 AG005681-29/AG/NIA NIH HHS/United States
- P01 AG026276-07/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical