Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction - PubMed (original) (raw)
Comparative Study
Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction
Caitlin A Orsini et al. J Neurosci. 2011.
Abstract
Knowing when and where to express fear is essential to survival. Recent work in fear extinction paradigms reveals that the contextual regulation of fear involves a neural network involving the hippocampus, medial prefrontal cortex, and amygdala. The amygdaloid basal nuclei (BA) receive convergent input from the ventral hippocampus (VH) and prelimbic (PL) prefrontal cortex and may integrate VH and PL input to regulate fear expression. To examine the functional organization of this neural circuit, we used cellular imaging of c-fos expression in anatomically defined neuronal populations and circuit disconnections to identify the pathways involved in the contextual control of extinguished fear. Before behavioral testing, we infused a retrograde tracer into the amygdala to label BA-projecting neurons in VH and PL. Rats then underwent fear conditioning and extinction and were tested for their fear to the extinguished conditioned stimulus (CS) in either the extinction context or in another context; freezing behavior served as the index of conditional fear. CS presentation outside the extinction context renewed conditional freezing and was associated with significantly more c-fos expression in BA-projecting neurons in the VH and PL than that induced by CS presentation in the extinction context. We next examined whether direct or indirect projections of VH to BA mediate fear renewal. Interestingly, disconnections of the VH from either the BA or PL eliminated renewal. These findings suggest that convergent inputs from both the VH and PL in the BA mediate the contextual control of fear after extinction.
Figures
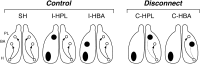
Figure 1.
A schematic of the projections between the hippocampus (H), BA, and PL in control rats and rats in which the projections were disconnected. In intact brains (SH), there are ipsilateral projections (arrows) from the VH (H) to both the BA and PL. Control rats with ipsilateral lesions of either the VH and PL (I-HPL) or the VH and BA (I-HBA) maintain connectivity between the hippocampus, amygdala, and PL in the intact hemisphere. However, contralateral lesions of the VH and PL (C-HPL) or VH and BA (C-HBA) disconnect the hippocampus (dashed lines) from the PL and BA, respectively.
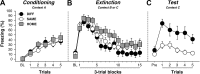
Figure 2.
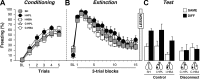
Conditioned freezing behavior in rats previously infused with CTb. A, Mean ± SEM percentage of freezing during fear conditioning, which consisted of a 3 min baseline period followed by five tone–shock pairings. Freezing was averaged across the pre-CS baseline (BL) as well as during each of the five conditioning trials; each trial consisted of the average of freezing during each CS presentation and the subsequent ISI. B, Mean ± SEM percentage of freezing during the 45 tone-alone extinction session. Freezing was averaged across the BL period as well as during the 45 extinction trials; as with conditioning, each trial consisted of the average of freezing during each CS presentation and subsequent ISI (data were binned into 15 blocks of three-trial averages). C, Mean ± SEM percentage of freezing during the test session that consisted of five tone-alone presentations with 30 s ISIs. Freezing was measured during the BL period and during the five trials, each of which consisted of a CS presentation and the subsequent ISI. Data are shown for rats that were tested outside the extinction context (DIFF; black circles), tested within the extinction context (SAME; white circles), or not tested at all (HOME; gray squares).
Figure 3.
Illustrations of the CTb injection site and CTb spread within the BA. A, A representative CTb-stained coronal section displaying the site of the CTb injection. B, Adjacent thionin sections were used to ensure that CTb spread did not extend beyond the boundaries of the BA. C, Schematic of CTb spread; gray indicates rats with maximal CTb spread, and black represents rats with the smallest injections of CTb. Images were adapted from Swanson (2004).
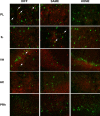
Figure 4.
Photomicrographs of representative double-labeled neurons in the PL, IL, VH, AC, and PRh for each behavioral group (DIFF, SAME, HOME). White arrows indicate double-labeled neurons; CTb-positive neurons are stained green, and c-fos-positive neurons are stained red.
Figure 5.
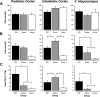
Quantification of CTb-positive, c-fos positive, and double-labeled neurons in the PL, IL, and VH. A, Mean ± SEM cell counts for CTb-positive neurons in the PL, IL, and VH. B, Mean ± SEM cell counts for c-fos-positive neurons in the PL, IL, and VH. C, Mean ± SEM percentage of double-labeled neurons (counts of CTB + c-fos-positive neurons divided by counts of CTB alone) in the PL, IL, and VH.
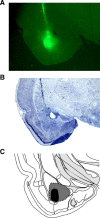
Figure 6.
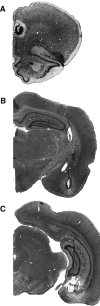
Photomicrographs of thionin-stained coronal sections showing representative lesions in the PL (A), BA (B), and VH (C) for three different rats in the disconnection experiment.
Figure 7.
Conditioned freezing in rats that received post-extinction VH, PL, or BA lesions. A, Mean ± SEM percentage of freezing during fear conditioning that consisted of a 3 min baseline period (BL) followed by five tone–shock presentations. Freezing was averaged across the pre-CS BL as well as during each of the five conditioning trials; each trial consisted of the average of freezing during each CS presentation and subsequent ISI. B, Mean ± SEM percentage of freezing during the 45 tone-alone extinction session. As with conditioning, freezing was averaged across the 3 min BL period as well as during the 45 trials, each of which consisted of a CS presentation and the subsequent ISI (data were binned into 15 blocks of three-trial averages). C, Mean ± SEM percentage of freezing during the first 5 min of the test session. Freezing was measured during the 3 min BL period as well as during the first five trials. Data are shown for rats that received sham surgeries (SH; gray squares), rats that received ipsilateral lesions of the VH and PL (I-HPL; black circles), ipsilateral lesions of the VH and BA (I-HBA; white squares), contralateral lesions of the VH and PL (C-HPL; white triangles), or contralateral lesions of the VH and BA (C-HBA; white circles).
Figure 8.
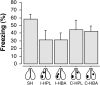
Conditioned freezing in rats that received VH, PL, or BA lesions after fear conditioning. During the test session, freezing was averaged across the baseline period as well as during the 45 trials, each of which consisted of a CS presentation and the subsequent ISI. The data are represented by the mean ± SEM percentage of freezing during the first 5 min of the test session.
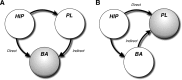
Figure 9.
Circuit model of hippocampal–prefrontal–amygdaloid interactions in the renewal of fear. A, In this scenario, both direct and indirect projections of the VH to the BA are required for the renewal of fear after extinction. Disconnection of either the direct or indirect pathways deprives the BA of convergent input from the VH and PL. B, Another possibility is that convergence of direct and indirect projections from the VH to the PL mediate the renewal of fear. Indeed, disconnection of either the VH–PL or VH–BA projection prevents convergence of VH and BA input in the PL.
Similar articles
- Renewal of extinguished fear activates ventral hippocampal neurons projecting to the prelimbic and infralimbic cortices in rats.
Wang Q, Jin J, Maren S. Wang Q, et al. Neurobiol Learn Mem. 2016 Oct;134 Pt A(Pt A):38-43. doi: 10.1016/j.nlm.2016.04.002. Epub 2016 Apr 7. Neurobiol Learn Mem. 2016. PMID: 27060752 Free PMC article. - Fear renewal preferentially activates ventral hippocampal neurons projecting to both amygdala and prefrontal cortex in rats.
Jin J, Maren S. Jin J, et al. Sci Rep. 2015 Feb 11;5:8388. doi: 10.1038/srep08388. Sci Rep. 2015. PMID: 25669753 Free PMC article. - Synaptic Targeting of Double-Projecting Ventral CA1 Hippocampal Neurons to the Medial Prefrontal Cortex and Basal Amygdala.
Kim WB, Cho JH. Kim WB, et al. J Neurosci. 2017 May 10;37(19):4868-4882. doi: 10.1523/JNEUROSCI.3579-16.2017. Epub 2017 Apr 6. J Neurosci. 2017. PMID: 28385873 Free PMC article. - Neural circuits involved in the renewal of extinguished fear.
Chen W, Wang Y, Wang X, Li H. Chen W, et al. IUBMB Life. 2017 Jul;69(7):470-478. doi: 10.1002/iub.1636. Epub 2017 May 2. IUBMB Life. 2017. PMID: 28464461 Review. - Prefrontal control of fear: more than just extinction.
Sotres-Bayon F, Quirk GJ. Sotres-Bayon F, et al. Curr Opin Neurobiol. 2010 Apr;20(2):231-5. doi: 10.1016/j.conb.2010.02.005. Epub 2010 Mar 18. Curr Opin Neurobiol. 2010. PMID: 20303254 Free PMC article. Review.
Cited by
- A model of amygdala-hippocampal-prefrontal interaction in fear conditioning and extinction in animals.
Moustafa AA, Gilbertson MW, Orr SP, Herzallah MM, Servatius RJ, Myers CE. Moustafa AA, et al. Brain Cogn. 2013 Feb;81(1):29-43. doi: 10.1016/j.bandc.2012.10.005. Epub 2012 Nov 17. Brain Cogn. 2013. PMID: 23164732 Free PMC article. - Treating the Developing versus Developed Brain: Translating Preclinical Mouse and Human Studies.
Casey BJ, Glatt CE, Lee FS. Casey BJ, et al. Neuron. 2015 Jun 17;86(6):1358-68. doi: 10.1016/j.neuron.2015.05.020. Neuron. 2015. PMID: 26087163 Free PMC article. Review. - Effects of Peripheral Immune Challenge on In Vivo Firing of Basolateral Amygdala Neurons in Adult Male Rats.
Munshi S, Rosenkranz JA. Munshi S, et al. Neuroscience. 2018 Oct 15;390:174-186. doi: 10.1016/j.neuroscience.2018.08.017. Epub 2018 Aug 29. Neuroscience. 2018. PMID: 30170159 Free PMC article. - The complexity of ventral CA1 and its multiple functionalities.
Hong I, Kaang BK. Hong I, et al. Genes Brain Behav. 2022 Sep;21(7):e12826. doi: 10.1111/gbb.12826. Epub 2022 Jul 11. Genes Brain Behav. 2022. PMID: 35815710 Free PMC article. Review. - Developmental rodent models of fear and anxiety: from neurobiology to pharmacology.
Ganella DE, Kim JH. Ganella DE, et al. Br J Pharmacol. 2014 Oct;171(20):4556-74. doi: 10.1111/bph.12643. Epub 2014 Jul 1. Br J Pharmacol. 2014. PMID: 24527726 Free PMC article. Review.
References
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. - PubMed
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5:368–378. - PubMed
Publication types
MeSH terms
Grants and funding
- F31 MH091822/MH/NIMH NIH HHS/United States
- R01MH065961/MH/NIMH NIH HHS/United States
- R01 MH065961/MH/NIMH NIH HHS/United States
- F31MH091822/MH/NIMH NIH HHS/United States
- R01 MH065961-09/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources