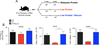Protein energy malnutrition impairs homeostatic proliferation of memory CD8 T cells - PubMed (original) (raw)
Protein energy malnutrition impairs homeostatic proliferation of memory CD8 T cells
Smita S Iyer et al. J Immunol. 2012.
Abstract
Nutrition is a critical but poorly understood determinant of immunity. There is abundant epidemiological evidence linking protein malnutrition to impaired vaccine efficacy and increased susceptibility to infections; yet, the role of dietary protein in immune memory homeostasis remains poorly understood. In this study, we show that protein-energy malnutrition induced in mice by low-protein (LP) feeding has a detrimental impact on CD8 memory. Relative to adequate protein (AP)-fed controls, LP feeding in lymphocytic choriomeningitis virus (LCMV)-immune mice resulted in a 2-fold decrease in LCMV-specific CD8 memory T cells. Adoptive transfer of memory cells, labeled with a division tracking dye, from AP mice into naive LP or AP mice demonstrated that protein-energy malnutrition caused profound defects in homeostatic proliferation. Remarkably, this defect occurred despite the lymphopenic environment in LP hosts. Whereas Ag-specific memory cells in LP and AP hosts were phenotypically similar, memory cells in LP hosts were markedly less responsive to polyinosinic-polycytidylic acid-induced acute proliferative signals. Furthermore, upon recall, memory cells in LP hosts displayed reduced proliferation and protection from challenge with LCMV-clone 13, resulting in impaired viral clearance in the liver. The findings show a metabolic requirement of dietary protein in sustaining functional CD8 memory and suggest that interventions to optimize dietary protein intake may improve vaccine efficacy in malnourished individuals.
Figures
Figure 1
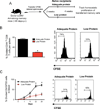
Effect of protein-energy malnutrition on LCMV-specific memory CD8 T cells. A) Experimental outline; 6–8 week-old B6 mice were infected intraperitoneally with LCMV Armstrong and after 40 days post-infection, mice were assigned to receive either an adequate protein (18% dietary protein) or a low protein diet (0.6% dietary protein). Analysis of LCMV-specific memory CD8 T cells in the spleen was performed after 4 weeks of dietary treatment. B) Db/GP276 and Db/GP33 tetramer staining of CD8 T cells from the spleen of adequate protein (top panel) and low protein-fed (bottom panel) mice. Data are percent of tetramer positive CD8 T cells. C) Absolute numbers of antigen-specific CD8 T cells in the spleen in adequate protein (black bars) and low protein-fed (red bars) mice. One representative experiment of three is shown. Data are mean of four mice per group + SEM; * P < 0.05
Figure 2
Effect of protein-energy malnutrition on cytokine production by LCMV-specific memory CD8 T cells. A) Intracellular cytokine staining for IFN-γ, TNF-a, and IL-2 after 5 h of GP276 peptide stimulation. Data are percent of CD8 T cells that produced cytokine. B) Median fluorescence intensity (MFI) of cytokine expression after GP276 stimulation. C) Total cytokine producing cells in the spleen after GP276 in adequate protein (black bars) and low protein-fed (red bars) mice. One representative experiment of three is shown. Data are mean of four mice per group+ SEM; * P < 0.05
Figure 3
Acute homeostatic proliferation of memory CD8 T cells in protein-energy malnutrition. A) Adequate protein and low protein-fed Armstrong immune mice were injected intraperitoneally with poly (I:C) and division of CD44hi CD8 T cells was determined 3 days later. (B) Percentage of CD44hi CD8s that responded to poly (I:C) in the spleen and bone marrow was assessed by intracellular staining with Ki-67. Histograms show median fluorescence intensity of Ki-67 in CD44hi CD8s T cells; grey dashed lines show Ki-67 staining in CD44lo CD8 T cells. One representative mouse of four from two experimental replicates is shown.
Figure 4
Basal homeostatic proliferation of memory CD8 T cells in protein-energy malnutrition. A) Splenocytes were prepared from LCMV-immune mice, labeled with CFSE, and adoptively transferred into naive B6 recipients receiving either an adequate protein or a low protein diet. Diets were continued for 4 weeks after cell transfer. (B) Homeostatic proliferation of donor cells was assessed in the spleen by examining CFSE dilution. Histogram shows % of transferred cells that divided. Bar graph shows total numbers of CD44hi T cells per spleen. In (C) donor cells were obtained Armstrong immune P14 chimeric mice. Histogram shows % of divided donor cells. Kinetic analysis of divided donor cells in blood is shown in the graph. One representative experiment of three is shown. Data are mean of three mice per group+ SEM; * P < 0.05
Figure 5
Protective immunity of memory CD8 T cells in protein energy malnutrition. A) To assess protective immunity of memory CD8 T cells in protein-energy malnutrition, memory cells from AP-fed P14 chimeric mouse were labeled with CFSE and transferred to naive adequate protein or low-protein fed recipients. Recipients were challenged with LCMV clone-13 and recall responses were assessed 7 days after transfer. B) shows a representative flow plot of percentage of Thy 1.1 + Db/GP33+ tetramer-specific CD8 T cells in adequate protein and low protein-fed hosts. Bar graph shows total Db/GP33 tetramer-specific CD8 T cells per spleen. In C) intracellular cytokine staining for IFN-γ and IL-2 after 5 h of GP33 peptide stimulation is shown. Data are percent of CD8 T cells that produced both IFN-γ and IL-2. In D) viral titers in liver and lung are shown. In a separate experiment (Figure 6A) malnourished memory cells from LP-fed mice were transferred to naive adequate protein or low protein- fed recipients subsequently challenged with VV-GP33. Recall responses were assessed 7 days after transfer. B) shows a representative flow plot of percentage of Thy 1.1+ Db/GP33+ tetramer-specific CD8 T cells in adequate protein and low-protein hosts. Total number of tetramer positive cells per spleen in shown in C. One representative experiment of three is shown. Data are mean of three mice per group+ SEM; * P < 0.05
Figure 6
Protective immunity of memory CD8 T cells in protein energy malnutrition. A) To assess protective immunity of memory CD8 T cells in protein-energy malnutrition, memory cells from AP-fed P14 chimeric mouse were labeled with CFSE and transferred to naive adequate protein or low-protein fed recipients. Recipients were challenged with LCMV clone-13 and recall responses were assessed 7 days after transfer. B) shows a representative flow plot of percentage of Thy 1.1 + Db/GP33+ tetramer-specific CD8 T cells in adequate protein and low protein-fed hosts. Bar graph shows total Db/GP33 tetramer-specific CD8 T cells per spleen. In C) intracellular cytokine staining for IFN-γ and IL-2 after 5 h of GP33 peptide stimulation is shown. Data are percent of CD8 T cells that produced both IFN-γ and IL-2. In D) viral titers in liver and lung are shown. In a separate experiment (Figure 6A) malnourished memory cells from LP-fed mice were transferred to naive adequate protein or low protein- fed recipients subsequently challenged with VV-GP33. Recall responses were assessed 7 days after transfer. B) shows a representative flow plot of percentage of Thy 1.1+ Db/GP33+ tetramer-specific CD8 T cells in adequate protein and low-protein hosts. Total number of tetramer positive cells per spleen in shown in C. One representative experiment of three is shown. Data are mean of three mice per group+ SEM; * P < 0.05
Figure 7
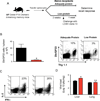
Protein supplementation rescues malnutrition-induced defect in CD8 T cell memory maintenance. A) Experimental outline; 6–8 week-old P14 Chimeric mice were infected intraperitoneally with LCMV Armstrong and 40 days post-infection, mice were assigned to receive either an adequate protein (18% dietary protein) or a low protein diet (0.6% dietary protein). A third group of mice received LP diet for 4 weeks followed by switch to AP diet. Analysis of LCMV-specific memory CD8 T cells in the spleen was performed after 8 weeks of dietary treatment. B) Db/GP33 tetramer staining of donor Thy1.1 + CD8 T cells from the spleen of low protein (top panel), adequate protein (middle panel) and low-protein rescue mice (bottom panel). Data are percent of tetramer positive donor CD8 T cells. C) Percentage, absolute numbers of GP33-specific donor CD8 T cells, and total CD44-high memory CD8 T cells in the spleen in adequate protein (black bars), low protein (red bars), and low protein rescue (blue bars) mice. One representative experiment of two is shown. Data are mean of four mice per group + SEM; * P < 0.05
Figure 8
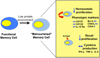
Protein-energy malnutrition impairs CD8 memory homeostasis. Malnourished memory CD8 T cells demonstrate markedly reduced homeostatic proliferation despite no measurable decrease in cell surface expression of the gamma chain cytokine receptors. Malnourished memory CD8 T also conferred reduced protective immunity due to impaired recall proliferation. The data show that dietary protein is a critical for sustaining a long-lived and functional memory CD8 T cell pool and that this defect may be rescued by protein supplementation.
Similar articles
- MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection.
Rahman AH, Cui W, Larosa DF, Taylor DK, Zhang J, Goldstein DR, Wherry EJ, Kaech SM, Turka LA. Rahman AH, et al. J Immunol. 2008 Sep 15;181(6):3804-10. doi: 10.4049/jimmunol.181.6.3804. J Immunol. 2008. PMID: 18768833 Free PMC article. - Homeostatic turnover of virus-specific memory CD8 T cells occurs stochastically and is independent of CD4 T cell help.
Choo DK, Murali-Krishna K, Anita R, Ahmed R. Choo DK, et al. J Immunol. 2010 Sep 15;185(6):3436-44. doi: 10.4049/jimmunol.1001421. Epub 2010 Aug 23. J Immunol. 2010. PMID: 20733203 - The role of CD80/CD86 in generation and maintenance of functional virus-specific CD8+ T cells in mice infected with lymphocytic choriomeningitis virus.
Grujic M, Bartholdy C, Remy M, Pinschewer DD, Christensen JP, Thomsen AR. Grujic M, et al. J Immunol. 2010 Aug 1;185(3):1730-43. doi: 10.4049/jimmunol.0903894. Epub 2010 Jul 2. J Immunol. 2010. PMID: 20601595 - IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections.
Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, Welsh RM. Bahl K, et al. J Immunol. 2006 Apr 1;176(7):4284-95. doi: 10.4049/jimmunol.176.7.4284. J Immunol. 2006. PMID: 16547266 - CD8 T cell responses to lymphocytic choriomeningitis virus in early growth response gene 1-deficient mice.
Singh A, Svaren J, Grayson J, Suresh M. Singh A, et al. J Immunol. 2004 Sep 15;173(6):3855-62. doi: 10.4049/jimmunol.173.6.3855. J Immunol. 2004. PMID: 15356133
Cited by
- Anorexia Nervosa and the Immune System-A Narrative Review.
Gibson D, Mehler PS. Gibson D, et al. J Clin Med. 2019 Nov 8;8(11):1915. doi: 10.3390/jcm8111915. J Clin Med. 2019. PMID: 31717370 Free PMC article. Review. - The effects of age and systemic metabolism on anti-tumor T cell responses.
Drijvers JM, Sharpe AH, Haigis MC. Drijvers JM, et al. Elife. 2020 Nov 10;9:e62420. doi: 10.7554/eLife.62420. Elife. 2020. PMID: 33170123 Free PMC article. Review. - Malnutrition drives infection susceptibility and dysregulated myelopoiesis that persists after refeeding intervention.
Sukhina A, Queriault C, Hall E, Rome K, Aggarwal M, Nunn E, Weiss A, Nguyen J, Bailis W. Sukhina A, et al. bioRxiv [Preprint]. 2024 Aug 19:2024.08.19.608575. doi: 10.1101/2024.08.19.608575. bioRxiv. 2024. PMID: 39229137 Free PMC article. Preprint. - A Nutrition-Related Factor-Based Risk Stratification for Exploring the Clinical Benefits in the Treatment of Patients With Locally Advanced Esophageal Squamous Cell Carcinoma Receiving Definitive Chemoradiotherapy: A Retrospective Cohort Study.
Yu Y, Wu H, Qiu J, Ke D, Wu Y, Lin M, Liu T, Zheng Q, Zheng H, Yang J, Wang Z, Li H, Liu L, Yao Q, Li J, Cheng W, Chen X. Yu Y, et al. Front Nutr. 2022 Aug 4;9:896847. doi: 10.3389/fnut.2022.896847. eCollection 2022. Front Nutr. 2022. PMID: 35990358 Free PMC article. - Malnutrition Decreases Antibody Secreting Cell Numbers Induced by an Oral Attenuated Human Rotavirus Vaccine in a Human Infant Fecal Microbiota Transplanted Gnotobiotic Pig Model.
Michael H, Langel SN, Miyazaki A, Paim FC, Chepngeno J, Alhamo MA, Fischer DD, Srivastava V, Kathayat D, Deblais L, Rajashekara G, Saif LJ, Vlasova AN. Michael H, et al. Front Immunol. 2020 Feb 14;11:196. doi: 10.3389/fimmu.2020.00196. eCollection 2020. Front Immunol. 2020. PMID: 32117313 Free PMC article.
References
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. - PubMed
- Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–225. - PubMed
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. - PubMed
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM008169/GM/NIGMS NIH HHS/United States
- N01AI30048/AI/NIAID NIH HHS/United States
- R37 AI030048/AI/NIAID NIH HHS/United States
- AI30048/AI/NIAID NIH HHS/United States
- R37 AI030048-10/AI/NIAID NIH HHS/United States
- R01 AI030048/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials