A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory - PubMed (original) (raw)
A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory
Andrea M Siegel et al. Immunity. 2011.
Abstract
STAT3 transcription factor signaling in specific T helper cell differentiation has been well described, although the broader roles for STAT3 in lymphocyte memory are less clear. Patients with autosomal-dominant hyper-IgE syndrome (AD-HIES) carry dominant-negative STAT3 mutations and are susceptible to a variety of bacterial and fungal infections. We found that AD-HIES patients have a cell-intrinsic defect in the number of central memory CD4(+) and CD8(+) T cells compared to healthy controls. Naive T cells from AD-HIES patients had lower expression of memory-related transcription factors BCL6 and SOCS3, a primary proliferation defect, and they failed to acquire central memory-like surface phenotypes in vitro. AD-HIES patients showed a decreased ability to control varicella zoster virus (VZV) and Epstein-Barr virus (EBV) latency, and T cell memory to both of these viruses was compromised. These data point to a specific role for STAT3 in human central memory T cell formation and in control of certain chronic viruses.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
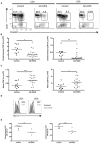
Figure 1. Patients with AD-HIES have fewer central memory T cells
A. The frequencies of naïve (CD27+CD45RO−), central (CD27+CD45RO+), and effector (CD27−CD45RO+/−) memory T cells were measured in AD-HIES patients and healthy age-matched controls by flow cytometry. Representative graphs and gating from age-matched subjects. Cells are gated singlet, aqua viability−CD3+. B. Absolute lymphocyte counts were used to calculate the number of central memory lymphocytes in each population. * p = 0.0083, ** p = 0.0036. C. A concomitant increase in the number of naïve (CD27+CD45RO−) T cells was observed in AD-HIES patients. Data are compiled from seven independent experiments. *** p = 0.0261, •p = 0.0160. D and E. CD127 expression on central memory (CD27+CD45RO+) T cells was measured by flow cytometry. D. Representative histograms of CD127 expression on CD27+CD45RO+ central memory T cells. E. Mean fluorescence intensity of CD127 on the surface of central memory AD-HIES T cells was lower than that of control cells •• p = 0.0317, ••• p = 0.0635. Data are representative of two similar experiments. All error bars represent median values, and significance calculated using a two-tailed Mann-Whitney t test with a 95% confidence interval.
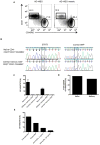
Figure 2. AD-HIES T cells have an intrinsic defect in memory differentiation
A. The frequency of central memory CD4+ T cells in a patient mosaic for an AD-HIES STAT3 mutation (G114ASTAT3) and their AD-HIES child. Cells are gated CD3+CD4+CD27+. Naïve (rectangle, CD27+CD31+CD45RO−) and central memory (circle, CD27+CD31−CD45RO+) mosaic AD-HIES T cells were purified by flow cytometry and the frequency of G1145ASTAT3 alleles determined by real-time PCR. B. Chromatographs of STAT3 and a control SNP (intron 13, rs2293152) depict the relative presence of mutant and normal STAT3 alleles in the naïve and memory CD4+ population. C. Naïve (CD31+CD27+CD45RO−), central (CD31−CD27+CD45RO+) and effector memory (CD31−CD27+CD45RO+/−) CD4+ and CD8+ T cells were sorted from the mosaic AD-HIES patient and subjected to sequence specific real-time PCR for the G114ASTAT3 allele. Real-time PCR measurements of mutant STAT3 alleles were measured in two independent experiments. D. Naïve (CD19+CD27lo) and memory (CD19+CD27hi) B cells were sorted from the AD-HIES mosaic patient and the frequency of cells with the G1145ASTAT3 allele was measured by real-time PCR. E. Naïve (CD31+CD27+CD45RO+) STAT3-mosaic CD4+ T cells were stimulated with irradiated feeder cells, PHA, with or without anti-IL-2Rα (Daclizumab) for ten days and mutant-STAT3 transcripts measured. Data is representative of two independent experiments.
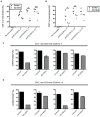
Figure 3. Decrease in central memory T cells in AD-HIES is not due to increased ex vivo apoptosis or decreased in vivo division
A. PBMCs from five AD-HIES and five healthy controls were thawed and cultured in complete RPMI for 48 hours without stimulation or exogenous cytokine. The frequency of AnnexinV+7AAD− cells was measured by flow cytometry in naïve (CD27+CD45RO−), central (CD27+CD45RO+), and effector (CD27−CD45RO+/−) memory CD4+ and CD8+ subsets. B to D. Thawed PBMCs were stained for intracellular Ki67 and gated on CD27+CD45RO+ (central) and CD27−CD45RO+/− (effector) T cells. B. Representative flow cytometry of Ki67 expression within CD4+ CD27+CD45RO+ central memory cells. Graphs depict singlet, aqua viability−CD3+CD4+CD27+CD45RO+ cells. C and D. Scatter plots depict Ki67 expression within central and effector T cell subsets of AD-HIES and control PBMCs from two independent experiments * p=0.0006, ** p =0.0401, *** p=0.0093. E. Serum levels of IL-2, IL-7, and IL-15 were measured by multiplex cytokine analysis. • p = 0.0014. All error bars represent median values, and significance calculated using a two-tailed Mann-Whitney t test with a 95% confidence interval.
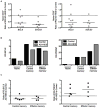
Figure 4. STAT3 mutant naïve T cells have impaired proliferation and in vitro induction of central memory T cell phenotypes
A. Sorted naïve (CD27−CD45RO−) CD4+ T cells were labeled with CFSE and cultured with anti-CD3 and anti-CD28 for five days in the presence of IL-2, IL-7, or IL-2 and IL-7. * p =0.05, ** p = 0.05, *** p = 0.05. B. Sorted naïve (CD27−CD45RO−) CD8+ T cells were labeled with CFSE and cultured with anti-CD3 and anti-CD28 for five days in the presence of IL-2, IL-15, or IL-2 and IL-15. • p = 0.05, •• p = 0.05. All error bars represent median values, and significance was calculated using a one-tailed Mann-Whitney t test with a 95% confidence interval. C and D. After four or five days, blasting or cells were analyzed for CD62L and CD45RO expression to determine differentiation status. Each bar graph represents and individual experiment with a unique control and AD-HIES pair. Different controls and AD-HIES patients were assayed in each experiment.
Figure 5. STAT3 mutant naïve T cells have decreased central memory-related transcription factor expression
A. Naïve (CD27+CD45RO−) CD4+ and CD8+ T cells were isolated from AD-HIES patients and controls by flow cytometry and transcript levels measured by real-time PCR. Each point represents the ratio of transcript observed in an AD-HIES patient compared to a corresponding healthy control subject. All data was collected from separate AD-HIES and control patients. B. Naïve (CD27+CD45RO−), central memory (CD27+CD45RO+), and effector memory (CD27−CD45RO+/−) CD8+ populations were purified by flow cytometry and BCL6 and PRDM1 transcripts were measured by real-time PCR. Each set of CD8+ populations was purified from a single individual. Data are representative of four independent experiments with four AD-HIES patients and four control subjects. C. Ratio of BCL6 and PRDM1 transcript levels between AD-HIES and control central and effector memory CD8+ T cell populations. Each point represents the ratio of transcript observed in an AD-HIES patient compared to their corresponding normal control.
Figure 6. AD-HIES patients have an increased risk for VZV reactivation and EBV viremia along with defects in virus-specific T cell memory
A. Kaplan-Meier plot of incidence of herpes zoster (VZV reactivation) in AD-HIES cohort. B. Thawed PBMCs were stimulated with inactivated homogenate from VZV infected cells and the sum of IFNγ, TNFα, and IL-2 production by total memory (CD27+CD45RO+ and CD27−CD45RO+/−) CD4+ T cells was measured relative to unstimulated cells. Data are composite of seven independent experiments. * p = 0.0127, ** p = 0.0089. C. The frequency of PBMCs harboring EBV genomes was determined by PCR assay. *** p = 0.0014. D. Thawed PBMCs were stimulated with inactivated lysate from EBV infected cells and IFNγ, TNFα, and IL-2 levels in total memory (CD27+CD45RO+ and CD27−CD45RO+/−) CD4+ and CD8+ T cells were measured by flow cytometry. E and F. T cells that produced TNFα and/or IFNγ in response to EBV were analyzed for their surface expression of CD27 and CD45RO. Data compiled from seven independent experiments. • p = 0.004, •• p = 0.0493. All error bars represent median values, and significance calculated using a two-tailed Mann-Whitney t test with a 95% confidence interval.
Comment in
- Keeping STATs on memory CD8+ T cells.
Olson JA, Jameson SC. Olson JA, et al. Immunity. 2011 Nov 23;35(5):663-5. doi: 10.1016/j.immuni.2011.11.006. Immunity. 2011. PMID: 22118521
Similar articles
- Signal transducer and activator of transcription 3 (STAT3) mutations underlying autosomal dominant hyper-IgE syndrome impair human CD8(+) T-cell memory formation and function.
Ives ML, Ma CS, Palendira U, Chan A, Bustamante J, Boisson-Dupuis S, Arkwright PD, Engelhard D, Averbuch D, Magdorf K, Roesler J, Peake J, Wong M, Adelstein S, Choo S, Smart JM, French MA, Fulcher DA, Cook MC, Picard C, Durandy A, Tsumura M, Kobayashi M, Uzel G, Casanova JL, Tangye SG, Deenick EK. Ives ML, et al. J Allergy Clin Immunol. 2013 Aug;132(2):400-11.e9. doi: 10.1016/j.jaci.2013.05.029. Epub 2013 Jul 4. J Allergy Clin Immunol. 2013. PMID: 23830147 Free PMC article. - Impaired memory B-cell development and antibody maturation with a skewing toward IgE in patients with STAT3 hyper-IgE syndrome.
van de Veen W, Krätz CE, McKenzie CI, Aui PM, Neumann J, van Noesel CJM, Wirz OF, Hagl B, Kröner C, Spielberger BD, Akdis CA, van Zelm MC, Akdis M, Renner ED. van de Veen W, et al. Allergy. 2019 Dec;74(12):2394-2405. doi: 10.1111/all.13969. Epub 2019 Aug 16. Allergy. 2019. PMID: 31269238 - Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation.
Siegel AM, Stone KD, Cruse G, Lawrence MG, Olivera A, Jung MY, Barber JS, Freeman AF, Holland SM, O'Brien M, Jones N, Nelson CG, Wisch LB, Kong HH, Desai A, Farber O, Gilfillan AM, Rivera J, Milner JD. Siegel AM, et al. J Allergy Clin Immunol. 2013 Dec;132(6):1388-96. doi: 10.1016/j.jaci.2013.08.045. Epub 2013 Nov 1. J Allergy Clin Immunol. 2013. PMID: 24184145 Free PMC article. - Clinical Manifestations and Genetic Analysis of 17 Patients with Autosomal Dominant Hyper-IgE Syndrome in Mainland China: New Reports and a Literature Review.
Wu J, Chen J, Tian ZQ, Zhang H, Gong RL, Chen TX, Hong L. Wu J, et al. J Clin Immunol. 2017 Feb;37(2):166-179. doi: 10.1007/s10875-017-0369-7. Epub 2017 Feb 14. J Clin Immunol. 2017. PMID: 28197791 Review. - Hyperimmunoglobulin E syndromes in pediatrics.
Zhang Q, Su HC. Zhang Q, et al. Curr Opin Pediatr. 2011 Dec;23(6):653-8. doi: 10.1097/MOP.0b013e32834c7f65. Curr Opin Pediatr. 2011. PMID: 21970826 Free PMC article. Review.
Cited by
- T cell vaccinology: exploring the known unknowns.
Burchill MA, Tamburini BA, Pennock ND, White JT, Kurche JS, Kedl RM. Burchill MA, et al. Vaccine. 2013 Jan 2;31(2):297-305. doi: 10.1016/j.vaccine.2012.10.096. Epub 2012 Nov 6. Vaccine. 2013. PMID: 23137843 Free PMC article. Review. - Targeting STAT3 in Cancer Immunotherapy.
Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Zou S, et al. Mol Cancer. 2020 Sep 24;19(1):145. doi: 10.1186/s12943-020-01258-7. Mol Cancer. 2020. PMID: 32972405 Free PMC article. Review. - The role of IL-27 in the induction of anti-tumor cytotoxic T lymphocyte response.
Liu Z, Yu J, Carson WE 3rd, Bai XF. Liu Z, et al. Am J Transl Res. 2013 Aug 15;5(5):470-80. eCollection 2013. Am J Transl Res. 2013. PMID: 23977407 Free PMC article. - Learning from Job: A Rare Genetic Disease and Lessons of Biblical Proportions.
Milner JD. Milner JD. Rambam Maimonides Med J. 2018 Jan 29;9(1):e0006. doi: 10.5041/RMMJ.10326. Rambam Maimonides Med J. 2018. PMID: 29406845 Free PMC article. Review. - Activating miRNA-mRNA network in gemcitabine-resistant pancreatic cancer cell associates with alteration of memory CD4+ T cells.
Gu J, Zhang J, Huang W, Tao T, Huang Y, Yang L, Yang J, Fan Y, Wang H. Gu J, et al. Ann Transl Med. 2020 Mar;8(6):279. doi: 10.21037/atm.2020.03.53. Ann Transl Med. 2020. PMID: 32355723 Free PMC article.
References
- Akaishi H, Takeda K, Kaisho T, Shineha R, Satomi S, Takeda J, Akira S. Defective IL-2-mediated IL-2 receptor alpha chain expression in Stat3-deficient T lymphocytes. Int Immunol. 1998;10:1747–1751. - PubMed
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. - PubMed
- Buckley RH, Schiff SE, Hayward AR. Reduced frequency of CD45RO+ T lymphocytes in blood of hyper-IgE syndrome patients. J Allergy Clin Immunol. 1991;87:313.
- Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous