Astroglial NF-κB mediates oxidative stress by regulation of NADPH oxidase in a model of retinal ischemia reperfusion injury - PubMed (original) (raw)
Comparative Study
Astroglial NF-κB mediates oxidative stress by regulation of NADPH oxidase in a model of retinal ischemia reperfusion injury
David J Barakat et al. J Neurochem. 2012 Feb.
Abstract
Astrocytes undergo rapid activation after injury, which is mediated in part by the transcription factor nuclear factor-kappaB (NF-κB). Consequently, activated astrocytes have been shown to induce the NF-κB regulated phagocyte NADPH oxidase (PHOX), resulting in elevated production of reactive oxygen species. We investigated the regulatory mechanisms of PHOX-induced oxidative stress in astrocytes and its non-cell-autonomous effects on retinal ganglion cell loss following retinal ischemia-reperfusion (IR) injury. To study PHOX activity and neurotoxicity mediated by glial NF-κB, we employed GFAP-IκBα-dn transgenic mice, where the NF-κB canonical pathway is suppressed specifically in astrocytes. Our analysis showed that NF-κB activation in astrocytes correlated with an increased expression of PHOX and reactive oxygen species production in primary cells and whole retinas subjected to oxygen-glucose deprivation or IR injury. Selective blockade of NF-κB in astrocytes or application of NADPH oxidase inhibitors suppressed retinal ganglion cell loss in co-cultures with astroglia challenged by oxygen-glucose deprivation. Furthermore, genetic suppression of astroglial NF-κB reduced oxidative stress in ganglion layer neurons in vivo in retinal IR. Collectively, our results suggest that astroglial NF-κB-regulated PHOX activity is a crucial toxicity pathway in the pathogenesis of retinal IR injury.
Published 2011. This article is a US Government work and is in the public domain in the USA.
Figures
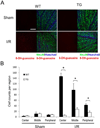
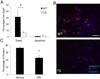
Figure 1. Inhibition of astroglial NF-κB reduces oxidative stress following retinal IR injury
(A) Representative images showing DNA/RNA damage localizing to NeuN-positive cells detected by 8-OH-guanosine (red) immunofluoresent labeling. Double-labeled cells are abundant in the GCL of the central retina 3 days after IR injury. Scale bar, 100 mm (B) Quantification of DNA/RNA damage; total counts of 8-OH-guanosine/NeuN/Hoechst-positive cells were averaged from the sum of 4 standard fields in each retinal region. Values are means ± SEM, n=6; *p<0.05.
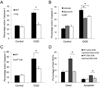
Figure 2. Inhibition of aNF-κB promotes survival of retinal ganglion cells in vitro after OGD
(A–C) Quantification of active caspase-3 positive RGCs in co-culture with astrocytes 24 hours after OGD challenge (OGD) vs. normoxic controls (Control) performed by confocal microscopy. The total quantities of NeuN or beta-III tubulin/Hoechst positive RGCs observed in 5 randomly selected fields of each experimental plate are compared. Values are means ± SEM, n=5; *p<0.05. (A) Activated caspase-3 positive RGCs in co-culture with WT and TG astrocytes; (B) Activated caspase-3 positive WT RGC in co-cultures with astrocytes treated with the NADPH oxidase inhibitors DPI (1µM) or apocynin (100µM); (C) Activated caspase-3 positive RGCs in co-cultures of WT astrocytes with WT or p47null RGCs (D) Cell death rates in WT and p47null RGCs challenged by astrocyte-conditioned media (ACM) from primary astrocytes subjected to OGD challenge (OGD ACM) or from normoxic control astrocytes (Control ACM). The percentages of dead (PI/Hoechst positive) and apoptotic (Annexin V/Hoechst positive) RGCs in ACM-treated cultures were calculated relative to total quantities of Hoechst positive cell in 5 randomly selected fields. Values are means ± SEM, n=5; *p<0.05.
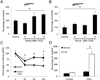
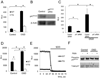
Figure 3. Transcriptional regulation of NADPH oxidase following OGD in primary astrocytes
(A–B) Gene expression analysis of the p47PHOX and gp91PHOX genes 6, 9 and 12 hours after OGD challenge. Changes were calculated as percentages of untreated control (Control) value. (C) Analysis of p47PHOXmRNA decay in actinomysin D (5µg/ml) treated cells with or without cycloheximide (5µg/ml) 12 hours after OGD treatment. (D) Relative abundance of p47PHOX transcript levels in astrocytes treated with vehicle or cycloheximide (5µg/ml). Gene expression levels were normalized to β-actin. Values are means ± SEM, n=4 and *p<0.05.
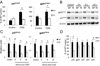
Figure 4. Regulation of homeostatic levels of PHOX is NF-κB dependent
(A) Gene expression analysis of gp91PHOX and p47PHOX transcripts 12 hours after OGD. Changes were calculated as percentages of untreated WT control (Control) values (n=5, *p<0.05). (B) Time course analysis of gp91PHOXand p47PHOXprotein levels after OGD by quantitative western blot. (C) Quantification of the data shown in (B): the gp91PHOX and p47PHOX band intensities were normalized to β-actin (n=3, *p<0.05). (D) Relative expression levels of genes encoding PHOX subunit in untreated WT and TG astrocytes (n=5; p<0.01).
Figure 5. ROS production by PHOX in astrocytes is regulated by NF-κB
(A) WT and TG astrocytes were assayed for superoxide production by Diogenes luminescence in control cultures and 24 hours after OGD challenge (OGD) (RLU = relative light units; n=5, p<0.05). (B) Transfection of p47PHOX siRNA reduces p47PHOX protein levels. Cell lysates were analyzed by western blot 24 hours post-transfection. (C) ROS production in live astrocytes was detected by Diogenes chemiluminescence 24 hours following OGD in non-targeting control siRNA (control) and p47PHOXsiRNA transfected cells. Astrocytes were transfected with siRNA 24 hours prior to OGD treatment. (n=5, *p<0.01). (D) Analysis of ROS production in WT and TG astrocytes 20 minutes after addition of PMA (1ng/ml) in control cultures and 24 hours after OGD (n=5, *p<0.01). (E) Representative recordings of ROS production from (D) in WT vs TG cells. Chemiluminesence was quenched by the addition of SOD (20U/ml). (F) Analysis of p47PHOX phosphorylation status by western blot in primary astrocyte extracts. OGD challenged and control astrocytes were treated with DMSO vehicle or PMA (1ng/ml) for 20 minutes prior to cell lysis.
Figure 6. The effects of NF-κB and PHOX suppression on OGD-induced cell death in primary astrocytes
(A–B) Cell death rates in cultures determined by quantification of cells with PI (red) and AnnexinV (green) labeling 24 hours after OGD (n=3, *p<0.05). (B) Representative images from experiment A showing abundant dead (PI-positive or PI/AnnexinV-positive) cells in WT but not in TG cultures; Hoechst dye (blue) labels nuclei for total cell counts. (C) Cell death rates (% of PI- positive among Hoechst- positive) cells in vehicle-treated control and 1µM DPI-treated astrocytes cultures 24 hours after OGD (n=4, *p<0.05). Scale bar 50µm.
Similar articles
- Transgenic inhibition of astroglial NF-κB protects from optic nerve damage and retinal ganglion cell loss in experimental optic neuritis.
Brambilla R, Dvoriantchikova G, Barakat D, Ivanov D, Bethea JR, Shestopalov VI. Brambilla R, et al. J Neuroinflammation. 2012 Sep 10;9:213. doi: 10.1186/1742-2094-9-213. J Neuroinflammation. 2012. PMID: 22963651 Free PMC article. - Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion.
Yokota H, Narayanan SP, Zhang W, Liu H, Rojas M, Xu Z, Lemtalsi T, Nagaoka T, Yoshida A, Brooks SE, Caldwell RW, Caldwell RB. Yokota H, et al. Invest Ophthalmol Vis Sci. 2011 Oct 11;52(11):8123-31. doi: 10.1167/iovs.11-8318. Invest Ophthalmol Vis Sci. 2011. PMID: 21917939 Free PMC article. - Inactivation of astroglial NF-kappa B promotes survival of retinal neurons following ischemic injury.
Dvoriantchikova G, Barakat D, Brambilla R, Agudelo C, Hernandez E, Bethea JR, Shestopalov VI, Ivanov D. Dvoriantchikova G, et al. Eur J Neurosci. 2009 Jul;30(2):175-85. doi: 10.1111/j.1460-9568.2009.06814.x. Epub 2009 Jul 9. Eur J Neurosci. 2009. PMID: 19614983 Free PMC article. - NADPH oxidases: novel therapeutic targets for neurodegenerative diseases.
Gao HM, Zhou H, Hong JS. Gao HM, et al. Trends Pharmacol Sci. 2012 Jun;33(6):295-303. doi: 10.1016/j.tips.2012.03.008. Epub 2012 Apr 11. Trends Pharmacol Sci. 2012. PMID: 22503440 Free PMC article. Review. - NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury.
Kleikers PW, Wingler K, Hermans JJ, Diebold I, Altenhöfer S, Radermacher KA, Janssen B, Görlach A, Schmidt HH. Kleikers PW, et al. J Mol Med (Berl). 2012 Dec;90(12):1391-406. doi: 10.1007/s00109-012-0963-3. Epub 2012 Oct 23. J Mol Med (Berl). 2012. PMID: 23090009 Review.
Cited by
- Transgenic inhibition of astroglial NF-κB protects from optic nerve damage and retinal ganglion cell loss in experimental optic neuritis.
Brambilla R, Dvoriantchikova G, Barakat D, Ivanov D, Bethea JR, Shestopalov VI. Brambilla R, et al. J Neuroinflammation. 2012 Sep 10;9:213. doi: 10.1186/1742-2094-9-213. J Neuroinflammation. 2012. PMID: 22963651 Free PMC article. - Retinal ganglion cell (RGC) programmed necrosis contributes to ischemia-reperfusion-induced retinal damage.
Dvoriantchikova G, Degterev A, Ivanov D. Dvoriantchikova G, et al. Exp Eye Res. 2014 Jun;123:1-7. doi: 10.1016/j.exer.2014.04.009. Epub 2014 Apr 19. Exp Eye Res. 2014. PMID: 24751757 Free PMC article. - Various Forms of Programmed Cell Death Are Concurrently Activated in the Population of Retinal Ganglion Cells after Ischemia and Reperfusion.
Dvoriantchikova G, Adis E, Lypka K, Ivanov D. Dvoriantchikova G, et al. Int J Mol Sci. 2023 Jun 8;24(12):9892. doi: 10.3390/ijms24129892. Int J Mol Sci. 2023. PMID: 37373037 Free PMC article. - Hypoxia-induced oxidative stress in ischemic retinopathy.
Li SY, Fu ZJ, Lo AC. Li SY, et al. Oxid Med Cell Longev. 2012;2012:426769. doi: 10.1155/2012/426769. Epub 2012 Oct 17. Oxid Med Cell Longev. 2012. PMID: 23125893 Free PMC article. Review. - Virally delivered, constitutively active NFκB improves survival of injured retinal ganglion cells.
Dvoriantchikova G, Pappas S, Luo X, Ribeiro M, Danek D, Pelaez D, Park KK, Ivanov D. Dvoriantchikova G, et al. Eur J Neurosci. 2016 Dec;44(11):2935-2943. doi: 10.1111/ejn.13383. Epub 2016 Sep 13. Eur J Neurosci. 2016. PMID: 27564592 Free PMC article.
References
- Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. - PubMed
- Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, Bethea JR. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182:2628–2640. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 EY017991-02/EY/NEI NIH HHS/United States
- P30 EY014801/EY/NEI NIH HHS/United States
- P30 EY014801-08/EY/NEI NIH HHS/United States
- R21EY020613/EY/NEI NIH HHS/United States
- EY017991/EY/NEI NIH HHS/United States
- R21 EY017991/EY/NEI NIH HHS/United States
- R21 EY020613/EY/NEI NIH HHS/United States
- R01 EY021517/EY/NEI NIH HHS/United States
- R21 EY020613-02/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous