MiR-106b impairs cholesterol efflux and increases Aβ levels by repressing ABCA1 expression - PubMed (original) (raw)
Comparative Study
MiR-106b impairs cholesterol efflux and increases Aβ levels by repressing ABCA1 expression
Jaekwang Kim et al. Exp Neurol. 2012 Jun.
Abstract
ATP-binding cassette transporter A1 (ABCA1) is a cholesterol transporter that transfers excess cellular cholesterol onto lipid-poor apolipoproteins. Given its critical role in cholesterol homeostasis, ABCA1 has been studied as a therapeutic target for Alzheimer's disease. Transcriptional regulation of ABCA1 by liver X receptor has been well characterized. However, whether ABCA1 expression is regulated at the posttranscriptional level is largely unknown. Identification of a novel pathway that regulates ABCA1 expression may provide new strategy for regulating cholesterol metabolism and amyloid β (Aβ) levels. Since ABCA1 has an unusually long 3' untranslated region, we investigated whether microRNAs could regulate ABCA1 expression. We identified miR-106b as a novel regulator of ABCA1 expression and Aβ metabolism. miR-106b significantly decreased ABCA1 levels and impaired cellular cholesterol efflux in neuronal cells. Furthermore, miR-106b dramatically increased levels of secreted Aβ by increasing Aβ production and preventing Aβ clearance. Alterations in Aβ production and clearance were rescued by expression of miR-106b-resistant ABCA1. Taken together, our data suggest that miR-106b affects Aβ metabolism by suppressing ABCA1 expression.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
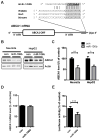
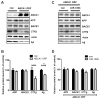
Fig. 1. miR-106b suppresses endogenous ABCA1 expression by directly targeting 3′ UTR of ABCA1
(A) Schematic diagram of ABCA1 mRNA containing conserved putative target site of miR-106b. The seed match in italic is indicated in the grey box. miR-106b binding region within 3′ UTR of ABCA1 is well conserved in human, rat, and mouse. (B) Decrease of endogenous ABCA1 levels by miR-106b in mouse neuroblastoma Neuro2a cells and human hepatocyte HepG2 cells. Cells were transfected with miR-106b or scrambled negative control. Forty eight hours post- transfection, cells were harvested for analyses. (C) ABCA1 protein levels were normalized by β-actin levels and quantified as a percentage of control. (D) Quantitative RT-PCR analysis of ABCA1 mRNA levels in Neuro2a cells. The levels of ABCA1 mRNA were not changed by miR-106b. GAPDH mRNA levels were used as a normalization control. (E) miR-106b suppressed expression of luciferase with full-length hABCA1 3′ UTR. Luciferase reporter construct used here contains the full-length 3′ UTR of hABCA1 at the downstream of luciferase. Luciferase reporter assay was done as described in the “Materials and Methods”. Data are shown as a percentage of negative control. Values are mean ± SEM. (*, p<0.05; **, p<0.01; ***, p<0.001 compared to the control).
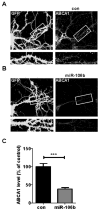
Fig. 2. miR-106b suppresses endogenous ABCA1 expression in rat primary neurons
Primary hippocampal neurons (DIV 14) were cotransfected with GFP vector and scrambled negative control (A), or GFP vector and 75 nM miR-106b (B). Forty eight hours post-transfection, cells were fixed and immunostained with anti-ABCA1 antibody. (C) The levels of ABCA1 on the dendrites were measured using MetaMorph® software. Data are shown as a percentage of negative control. Values are mean ± SEM. (***, p<0.001 compared to the control).
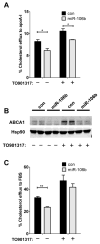
Fig. 3. miR-106b decreases cellular cholesterol efflux
Neuro2a cells were transfected with miR-106b or scrambled negative control for 24hr and then incubated the cells with radioactively labeled 3H-cholesterol. After 24hr, cells were incubated with human apoA-I (A) or FBS (C) as a cholesterol acceptor with or without LXR agonist (TO901317), as described in the “Materials and Methods”. Data is shown as a percentage of total cellular 3H-cholesterol content (total effluxed 3H-cholesterol+cell-associated 3H-cholesterol). (B) ABCA1 protein levels were monitored by western blot analysis. While TO091317 treatment significantly increased ABCA1 protein levels compared with no treatment, miR-106b dramatically repressed TO901317-mediated ABCA1 induction.
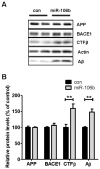
Fig. 4. miR-106b increases levels of secreted Aβ
Neuro2a cells were first transfected with hAPPsw construct for 8h and allowed to recover overnight. miR-106b or scrambled negative control was transfected into cells. Forty eight hours post-transfection, cells were incubated in fresh medium. 6h after media change, cells and media were collected and analyzed. (A) Western blot analysis of APP, BACE1, CTFβ, and Aβ in Neuro2a cells transfected with miR-106b or negative control. miR-106b did not alter levels of APP and BACE1 but significantly increased the levels of cellular CTFβ and secreted Aβ. Aβ levels were measured in media and all other proteins were detected in cell lysates. (B) Quantification of APP, BACE1, CTFβ, and Aβ levels. The levels of APP and BACE1 were normalized with the actin levels. The levels of CTFβ and Aβ were normalized with the APP levels. Data is shown as a percentage of control and values are mean ± SEM. (**p<0.01 compared to the control).
Fig. 5. Alterations in CTFβ and Aβ by miR-106b were rescued by overexpression of ABCA1 ORF
(A) ABCA1 decreased levels of cellular CTFβ and secreted Aβ. Neuro2a cells were first transfected with hAPPsw for 8h. Twenty four hours after the initial transfection, mABCA1 ORF construct or negative control vector was transfected into cells. After 48hr, cells were incubated in fresh medium. 6h later, cells and media were collected for analyses. Aβ levels in media and all other protein levels in cell lysates were analyzed by western blot. (B) Each protein level was quantified after normalization as a percentage of control. (C) Overexpression of ABCA1 ORF rescued the altered levels of CTFβ and secreted Aβ by miR-106b. Cells were first co-transfected with hAPPsw and mABCA1 ORF and then miR-106b or scrambled negative control was transfected into cells. Following procedures were same as above. (D) The levels of APP and BACE1 were normalized with the actin levels and the levels of CTFβ and Aβ were normalized with the APP levels. Values are mean ± SEM. (*, p<0.05; **, p<0.01 compared to the control).
Fig. 6. Reduction of Aβ clearance by miR-106b is rescued by ABCA1 ORF expression
(A) Reduction of Aβ clearance by miR-106b. Neuro2a cells were transfected with miR-106b or scrambled negative control. After 48hr, cells were incubated in fresh DMEM/10% FBS media containing 20 nM Aβ40. Twenty four hours later, both cells and media were collected for analyses. Aβ levels in media were analyzed by western blot. (B) Aβ levels were quantified after normalization as a percentage of control. (C) Overexpression of ABCA1 ORF rescued impairment of Aβ clearance by miR-106b. Cells were first transfected with mABCA1 ORF for 8hr. Twenty four hours after transfection, scrambled negative control or miR-106b was transfected into cells. Following procedures were same as above. (D) The levels of Aβ were normalized with total protein levels. (E) No change of Aβ clearance by miR-106b under FBS free condition. Neuro2a cells were transfected with scrambled negative control or miR-106b. After 48hr, cells were incubated in fresh DMEM/N2 media containing 20 nM Aβ40. Aβ clearance was not altered by miR-106b in the media without FBS (F). Values represent mean ± SEM. (**, p<0.01 compared to the control).
Similar articles
- microRNA-33 Regulates ApoE Lipidation and Amyloid-β Metabolism in the Brain.
Kim J, Yoon H, Horie T, Burchett JM, Restivo JL, Rotllan N, Ramírez CM, Verghese PB, Ihara M, Hoe HS, Esau C, Fernández-Hernando C, Holtzman DM, Cirrito JR, Ono K, Kim J. Kim J, et al. J Neurosci. 2015 Nov 4;35(44):14717-26. doi: 10.1523/JNEUROSCI.2053-15.2015. J Neurosci. 2015. PMID: 26538644 Free PMC article. - MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1.
Ramirez CM, Dávalos A, Goedeke L, Salerno AG, Warrier N, Cirera-Salinas D, Suárez Y, Fernández-Hernando C. Ramirez CM, et al. Arterioscler Thromb Vasc Biol. 2011 Nov;31(11):2707-14. doi: 10.1161/ATVBAHA.111.232066. Arterioscler Thromb Vasc Biol. 2011. PMID: 21885853 Free PMC article. - The effects of ABCA1 on cholesterol efflux and Abeta levels in vitro and in vivo.
Burns MP, Vardanian L, Pajoohesh-Ganji A, Wang L, Cooper M, Harris DC, Duff K, Rebeck GW. Burns MP, et al. J Neurochem. 2006 Aug;98(3):792-800. doi: 10.1111/j.1471-4159.2006.03925.x. Epub 2006 Jun 12. J Neurochem. 2006. PMID: 16771834 - Posttranscriptional regulation of ATP-binding cassette transporter A1 in lipid metabolism.
Lv YC, Yin K, Fu YC, Zhang DW, Chen WJ, Tang CK. Lv YC, et al. DNA Cell Biol. 2013 Jul;32(7):348-58. doi: 10.1089/dna.2012.1940. Epub 2013 May 24. DNA Cell Biol. 2013. PMID: 23705956 Review. - The role of ATP-binding cassette transporter A1 in Alzheimer's disease and neurodegeneration.
Koldamova R, Fitz NF, Lefterov I. Koldamova R, et al. Biochim Biophys Acta. 2010 Aug;1801(8):824-30. doi: 10.1016/j.bbalip.2010.02.010. Epub 2010 Feb 24. Biochim Biophys Acta. 2010. PMID: 20188211 Free PMC article. Review.
Cited by
- The Role of the miR-17-92 Cluster in Autophagy and Atherosclerosis Supports Its Link to Lysosomal Storage Diseases.
Ortuño-Sahagún D, Enterría-Rosales J, Izquierdo V, Griñán-Ferré C, Pallàs M, González-Castillo C. Ortuño-Sahagún D, et al. Cells. 2022 Sep 26;11(19):2991. doi: 10.3390/cells11192991. Cells. 2022. PMID: 36230953 Free PMC article. Review. - Nourin-Dependent miR-137 and miR-106b: Novel Early Inflammatory Diagnostic Biomarkers for Unstable Angina Patients.
Elgebaly SA, Christenson RH, Kandil H, El-Khazragy N, Rashed L, Yacoub B, Eldeeb H, Ali M, Sharafieh R, Klueh U, Kreutzer DL. Elgebaly SA, et al. Biomolecules. 2021 Feb 28;11(3):368. doi: 10.3390/biom11030368. Biomolecules. 2021. PMID: 33670982 Free PMC article. - The role of the microRNA regulatory network in Alzheimer's disease: a bioinformatics analysis.
Sun C, Liu J, Duan F, Cong L, Qi X. Sun C, et al. Arch Med Sci. 2021 Mar 18;18(1):206-222. doi: 10.5114/aoms/80619. eCollection 2022. Arch Med Sci. 2021. PMID: 35154541 Free PMC article. - microRNAs in Neurodegeneration: Current Findings and Potential Impacts.
Sharma S, Lu HC. Sharma S, et al. J Alzheimers Dis Parkinsonism. 2018;8(1):420. doi: 10.4172/2161-0460.1000420. Epub 2018 Jan 23. J Alzheimers Dis Parkinsonism. 2018. PMID: 29862137 Free PMC article. - MicroRNA control of high-density lipoprotein metabolism and function.
Rayner KJ, Moore KJ. Rayner KJ, et al. Circ Res. 2014 Jan 3;114(1):183-92. doi: 10.1161/CIRCRESAHA.114.300645. Circ Res. 2014. PMID: 24385511 Free PMC article. Review.
References
- Betel D, et al. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149–53. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL107953/HL/NHLBI NIH HHS/United States
- R03 AG034253/AG/NIA NIH HHS/United States
- R01HL107953/HL/NHLBI NIH HHS/United States
- AG034253/AG/NIA NIH HHS/United States
- P30NS069329/NS/NINDS NIH HHS/United States
- P30 NS069329/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases