GlcNAcylation of histone H2B facilitates its monoubiquitination - PubMed (original) (raw)
. 2011 Nov 27;480(7378):557-60.
doi: 10.1038/nature10656.
Waka Hashiba, Hiroki Sekine, Atsushi Yokoyama, Toshihiro Chikanishi, Saya Ito, Yuuki Imai, Jaehoon Kim, Housheng Hansen He, Katsuhide Igarashi, Jun Kanno, Fumiaki Ohtake, Hirochika Kitagawa, Robert G Roeder, Myles Brown, Shigeaki Kato
Affiliations
- PMID: 22121020
- PMCID: PMC7289526
- DOI: 10.1038/nature10656
GlcNAcylation of histone H2B facilitates its monoubiquitination
Ryoji Fujiki et al. Nature. 2011.
Abstract
Chromatin reorganization is governed by multiple post-translational modifications of chromosomal proteins and DNA. These histone modifications are reversible, dynamic events that can regulate DNA-driven cellular processes. However, the molecular mechanisms that coordinate histone modification patterns remain largely unknown. In metazoans, reversible protein modification by O-linked N-acetylglucosamine (GlcNAc) is catalysed by two enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA). However, the significance of GlcNAcylation in chromatin reorganization remains elusive. Here we report that histone H2B is GlcNAcylated at residue S112 by OGT in vitro and in living cells. Histone GlcNAcylation fluctuated in response to extracellular glucose through the hexosamine biosynthesis pathway (HBP). H2B S112 GlcNAcylation promotes K120 monoubiquitination, in which the GlcNAc moiety can serve as an anchor for a histone H2B ubiquitin ligase. H2B S112 GlcNAc was localized to euchromatic areas on fly polytene chromosomes. In a genome-wide analysis, H2B S112 GlcNAcylation sites were observed widely distributed over chromosomes including transcribed gene loci, with some sites co-localizing with H2B K120 monoubiquitination. These findings suggest that H2B S112 GlcNAcylation is a histone modification that facilitates H2BK120 monoubiquitination, presumably for transcriptional activation.
Figures
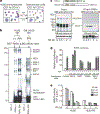
Figure 1 |. MLL5 acts as a co-activator of RARα.
a, Giemsa–May–Grunwald staining and cell-surface marker of HL60 cells. b, Outline (top) and silver-staining analysis (bottom) of the purification using GST-fused RARα (LBD) as bait. Diff. HL60, differentiated HL60 cells. c, Interaction between RARα and MLL5 during RA-induced differentiation. Protein structure of MLL5 (top). The anti-RARα immunoprecipitates (IP) from the RA-treated cells were subjected to western blotting (WB, bottom). The asterisk indicates a nonspecific band. d, Luciferase assay of MLL5 function in RAR-mediated transcription. RARE, RA response element. e, RARα-associating HKMT activities during RA-induced differentiation. The anti-RARα immunoprecipitants from the differentiating cells were used for in vitro HKMT assays with H3 tail peptides (1–21) and the indicated point-mutated peptides. *P < 0.05 versus the activity for the 1–21 peptide. Error bars, means and s.d. (n = 3). d.p.m., disintegrations per minute.
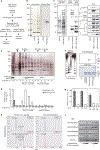
Figure 2 |. Purification of the HKMT-active MLL5 complex.
a, Outline of the purification. b, c, Silver staining and western blot analysis of the anti-Flag affinity purification for the Flag–MLL5 immunocomplex (b) and the gel-filtrated fractions (c). d, HKMT activities of the gel-filtrated fractions using the indicated tail peptides. e, Silver staining and western blot (WB) analysis of the MLL5-L and the MLL5-S complexes. The asterisk indicates a nonspecific band. f, g, Substrate preference of the MLL5-L complex. In vitro HKMT assay using the native histone substrates (f) and H3 tail peptides (g). In vitro reconstituted nucleosomes were analysed by micrococcal nuclease assay (MNase assay; f). CBB coomassie brilliant blue. h, i, Mass-spectrometric analysis ofthe H3 tail (1–21 and K4A) peptides (h) and western blot analysis of the nucleosomes (i) methylated by the MLL5-L complex. The asterisks indicate nonspecific peaks (h). Error bars, means and s.d. (n = 3). SAM, _S_-adenosyl-L-methionine.
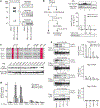
Figure 3 |. MLL5 is a GlcNAcylation-dependent HKMT.
a, Western blot (WB) analysis for GlcNAcylation of the MLL5-L and the MLL5-S complexes. The anti-GlcNAc bands were re-probed with the indicated antibodies. b, Effect of in vitro GlcNAcylation of the MLL5-L complex on its HKMT activity. The experimental procedure is summarized (left). Gal, UDP–GalNAc; Glc, UDP–GlcNAc. c, Mapping of GlcNAcylation sites of MLL5. Sequence alignment of MLL-family SET domains (top). The residues required for SAM binding are shaded in red. Western blot analysis for GlcNAcylation of the serial point-mutated MLL5s (indicated Ser/Thr to Ala, bottom). d, e, GlcNAc-modified (d) or -depleted (e) MLL5 mutants were subjected to the HKMT assay. GlcNAc levels were analysed by western blot (e). Error bars, means and s.d. (n = 3).
Figure 4 |. GlcNAcylation of MLL5 facilitates RA-induced granulopoiesis.
a, b, Effect of cellular GlcNAcylation on RA-induced granulopoiesis. The HL60 cells (a) or the HL60-R2 cells (b), exposed to the indicated reagents, were analysed by flow cytometry. The dashed line shows RA-untreated control (a). c, Western blot (WB) analysis for histone H3K4 methylation in the cells treated with RA, PUGNAc or both. d, Western blot analysis for GlcNAcylation of MLL5 and OGT in HL60 and HL60-R2 cells. e, Quantitative polymerase chain reaction (qPCR) analysis of CEBPE expression in the cells retrovirally transduced with the indicated shRNAs or expression vectors (using 18S rRNA as internal control). f, g, The roles of MLL5 and OGT on RA-induced differentiation. RA-induced differentiation of the HL60 cells, virally expressed with the indicated shRNAs (f) or the indicated constructs (g), were analysed by flow cytometry. shLuc (f) or Flag (g) control is overlaid (blue-open). Error bars, means and s.d. ( n = 3).
Similar articles
- Undetectable histone O-GlcNAcylation in mammalian cells.
Gagnon J, Daou S, Zamorano N, Iannantuono NV, Hammond-Martel I, Mashtalir N, Bonneil E, Wurtele H, Thibault P, Affar el B. Gagnon J, et al. Epigenetics. 2015;10(8):677-91. doi: 10.1080/15592294.2015.1060387. Epigenetics. 2015. PMID: 26075789 Free PMC article. - OGT mediated histone H2B S112 GlcNAcylation regulates DNA damage response.
Wang P, Peng C, Liu X, Liu H, Chen Y, Zheng L, Han B, Pei H. Wang P, et al. J Genet Genomics. 2015 Sep 20;42(9):467-75. doi: 10.1016/j.jgg.2015.07.002. Epub 2015 Jul 23. J Genet Genomics. 2015. PMID: 26408091 - Modification of histones by sugar β-N-acetylglucosamine (GlcNAc) occurs on multiple residues, including histone H3 serine 10, and is cell cycle-regulated.
Zhang S, Roche K, Nasheuer HP, Lowndes NF. Zhang S, et al. J Biol Chem. 2011 Oct 28;286(43):37483-95. doi: 10.1074/jbc.M111.284885. Epub 2011 Sep 6. J Biol Chem. 2011. PMID: 21896475 Free PMC article. - A critical perspective of the diverse roles of O-GlcNAc transferase in chromatin.
Gambetta MC, Müller J. Gambetta MC, et al. Chromosoma. 2015 Dec;124(4):429-42. doi: 10.1007/s00412-015-0513-1. Epub 2015 Apr 18. Chromosoma. 2015. PMID: 25894967 Free PMC article. Review. - Potential coordination role between O-GlcNAcylation and epigenetics.
Wu D, Cai Y, Jin J. Wu D, et al. Protein Cell. 2017 Oct;8(10):713-723. doi: 10.1007/s13238-017-0416-4. Epub 2017 May 9. Protein Cell. 2017. PMID: 28488246 Free PMC article. Review.
Cited by
- O-GlcNAc transferase invokes nucleotide sugar pyrophosphate participation in catalysis.
Schimpl M, Zheng X, Borodkin VS, Blair DE, Ferenbach AT, Schüttelkopf AW, Navratilova I, Aristotelous T, Albarbarawi O, Robinson DA, Macnaughtan MA, van Aalten DM. Schimpl M, et al. Nat Chem Biol. 2012 Dec;8(12):969-74. doi: 10.1038/nchembio.1108. Epub 2012 Oct 28. Nat Chem Biol. 2012. PMID: 23103942 Free PMC article. - Enhanced transfer of a photocross-linking N-acetylglucosamine (GlcNAc) analog by an O-GlcNAc transferase mutant with converted substrate specificity.
Rodriguez AC, Yu SH, Li B, Zegzouti H, Kohler JJ. Rodriguez AC, et al. J Biol Chem. 2015 Sep 11;290(37):22638-48. doi: 10.1074/jbc.M115.667006. Epub 2015 Aug 3. J Biol Chem. 2015. PMID: 26240142 Free PMC article. - Molecular Regulation of the Polycomb Repressive-Deubiquitinase.
Reddington CJ, Fellner M, Burgess AE, Mace PD. Reddington CJ, et al. Int J Mol Sci. 2020 Oct 22;21(21):7837. doi: 10.3390/ijms21217837. Int J Mol Sci. 2020. PMID: 33105797 Free PMC article. Review. - Role of novel histone modifications in cancer.
Shanmugam MK, Arfuso F, Arumugam S, Chinnathambi A, Jinsong B, Warrier S, Wang LZ, Kumar AP, Ahn KS, Sethi G, Lakshmanan M. Shanmugam MK, et al. Oncotarget. 2017 Dec 17;9(13):11414-11426. doi: 10.18632/oncotarget.23356. eCollection 2018 Feb 16. Oncotarget. 2017. PMID: 29541423 Free PMC article. Review. - Induced DNA demethylation by targeting Ten-Eleven Translocation 2 to the human ICAM-1 promoter.
Chen H, Kazemier HG, de Groote ML, Ruiters MH, Xu GL, Rots MG. Chen H, et al. Nucleic Acids Res. 2014 Feb;42(3):1563-74. doi: 10.1093/nar/gkt1019. Epub 2013 Nov 4. Nucleic Acids Res. 2014. PMID: 24194590 Free PMC article.
References
- Strahl BD & Allis CD The language of covalent histone modifications. Nature 403, 41–45 (2000). - PubMed
- Bernstein BE, Meissner A & Lander ES The mammalian epigenome. Cell 128, 669–681 (2007). - PubMed
- Sarma K & Reinberg D Histone variants meet their match. Nature Rev. Mol. Cell Biol 6, 139–149 (2005). - PubMed
- Li B, Carey M & Workman JL The role of chromatin during transcription. Cell 128, 707–719 (2007). - PubMed
- Zhang Y & Reinberg D Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 15, 2343–2360 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous