Angiotensin-converting enzyme 2: the first decade - PubMed (original) (raw)
Angiotensin-converting enzyme 2: the first decade
Nicola E Clarke et al. Int J Hypertens. 2012.
Abstract
The renin-angiotensin system (RAS) is a critical regulator of hypertension, primarily through the actions of the vasoactive peptide Ang II, which is generated by the action of angiotensin-converting enzyme (ACE) mediating an increase in blood pressure. The discovery of ACE2, which primarily metabolises Ang II into the vasodilatory Ang-(1-7), has added a new dimension to the traditional RAS. As a result there has been huge interest in ACE2 over the past decade as a potential therapeutic for lowering blood pressure, especially elevation resulting from excess Ang II. Studies focusing on ACE2 have helped to reveal other actions of Ang-(1-7), outside vasodilation, such as antifibrotic and antiproliferative effects. Moreover, investigations focusing on ACE2 have revealed a variety of roles not just catalytic but also as a viral receptor and amino acid transporter. This paper focuses on what is known about ACE2 and its biological roles, paying particular attention to the regulation of ACE2 expression. In light of the entrance of human recombinant ACE2 into clinical trials, we discuss the potential use of ACE2 as a therapeutic and highlight some pertinent questions that still remain unanswered about ACE2.
Figures
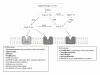
Figure 1
Schematic representation of the renin-angiotensin system (RAS). ACE: angiotensin-converting enzyme; ACE2: angiotensin-converting enzyme 2; NEP: neprilysin; AT1R: Ang II type 1 receptor. Angiotensinogen is cleaved by renin in the circulation to generate Ang I. Ang I is cleaved to yield Ang II by ACE, Ang-(1-7) by NEP, or Ang (1-9) by ACE2; this reaction is much less favourable than the production of Ang-(1-7) from Ang II. Ang-(1-9) is then cleaved by either NEP or ACE to yield Ang-(1-7) in a minor pathway. Ang II exerts its main actions by binding to the AT1R. Ang II can also be further cleaved by ACE2, into Ang-(1-7), which exerts its effects through its receptor (Mas). The opposing actions of the two receptors are listed above.
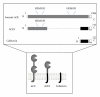
Figure 2
The domain structure and membrane topology of somatic ACE, ACE2, and collectrin. Each protein is a type I integral-membrane protein with an N-terminal ectodomain, a transmembrane region, and a short C-terminal cytoplasmic tail. Residue numbers are indicated. Both ACE and ACE2 contain zinc-binding motifs (HEMGH), which form the active sites of the enzyme: somatic ACE has two active sites whereas ACE2 only has one. Collectrin contains no catalytic residues. ACE2 is homologous to the N-terminal ectodomain of ACE but has no homology with its C-terminal cytoplasmic domain. Instead, it shares a number of residues with the intracellular domain of collectrin. Signal peptide in light grey; transmembrane domain in textured grey.
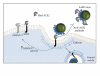
Figure 3
ACE2 acts as the host cell receptor for SARS-CoV, by binding to the spike protein on the viral capsid. Binding to ACE2 stimulates clathrin-dependent endocytosis of both ACE2 and the SARS-CoV, which is essential for viral infection. Binding of the spike protein to ACE2 induces ADAM 17 activity, thereby reducing the amount of ACE2 expressed on the cell surface. Treatment with soluble ACE-2 or anti-ACE-2 antibodies disrupts the interaction between virus and receptor.
Similar articles
- ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension.
Mendoza-Torres E, Oyarzún A, Mondaca-Ruff D, Azocar A, Castro PF, Jalil JE, Chiong M, Lavandero S, Ocaranza MP. Mendoza-Torres E, et al. Ther Adv Cardiovasc Dis. 2015 Aug;9(4):217-37. doi: 10.1177/1753944715597623. Epub 2015 Aug 13. Ther Adv Cardiovasc Dis. 2015. PMID: 26275770 Review. - Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy.
Oudit GY, Penninger JM. Oudit GY, et al. Curr Heart Fail Rep. 2011 Sep;8(3):176-83. doi: 10.1007/s11897-011-0063-7. Curr Heart Fail Rep. 2011. PMID: 21611889 Review. - ACE2: a new target for cardiovascular disease therapeutics.
Raizada MK, Ferreira AJ. Raizada MK, et al. J Cardiovasc Pharmacol. 2007 Aug;50(2):112-9. doi: 10.1097/FJC.0b013e3180986219. J Cardiovasc Pharmacol. 2007. PMID: 17703127 Review. - Discovery and characterization of ACE2 - a 20-year journey of surprises from vasopeptidase to COVID-19.
Hooper NM, Lambert DW, Turner AJ. Hooper NM, et al. Clin Sci (Lond). 2020 Sep 30;134(18):2489-2501. doi: 10.1042/CS20200476. Clin Sci (Lond). 2020. PMID: 32990314 - Cardiovascular protection by angiotensin-converting enzyme 2: a new paradigm.
Ferreira AJ, Hernández Prada JA, Ostrov DA, Raizada MK. Ferreira AJ, et al. Future Cardiol. 2008 Mar;4(2):175-82. doi: 10.2217/14796678.4.2.175. Future Cardiol. 2008. PMID: 19804294
Cited by
- Comparison of losartan and amlodipine effects on the outcomes of patient with COVID-19 and primary hypertension: A randomised clinical trial.
Nouri-Vaskeh M, Kalami N, Zand R, Soroureddin Z, Varshochi M, Ansarin K, Rezaee H, Taghizadieh A, Sadeghi A, Ahangari Maleki M, Esmailnajad A, Saleh P, Haghdoost M, Maleki M, Sharifi A. Nouri-Vaskeh M, et al. Int J Clin Pract. 2021 Jun;75(6):e14124. doi: 10.1111/ijcp.14124. Epub 2021 Mar 13. Int J Clin Pract. 2021. PMID: 33650197 Free PMC article. Clinical Trial. - Matrix metallopeptidase 9 as a host protein target of chloroquine and melatonin for immunoregulation in COVID-19: A network-based meta-analysis.
Hazra S, Chaudhuri AG, Tiwary BK, Chakrabarti N. Hazra S, et al. Life Sci. 2020 Sep 15;257:118096. doi: 10.1016/j.lfs.2020.118096. Epub 2020 Jul 15. Life Sci. 2020. PMID: 32679150 Free PMC article. - Significant Unresolved Questions and Opportunities for Bioengineering in Understanding and Treating COVID-19 Disease Progression.
Shirazi J, Donzanti MJ, Nelson KM, Zurakowski R, Fromen CA, Gleghorn JP. Shirazi J, et al. Cell Mol Bioeng. 2020 Jul 27;13(4):259-284. doi: 10.1007/s12195-020-00637-w. eCollection 2020 Aug. Cell Mol Bioeng. 2020. PMID: 32837585 Free PMC article. - A narrative review of the potential pharmacological influence and safety of ibuprofen on coronavirus disease 19 (COVID-19), ACE2, and the immune system: a dichotomy of expectation and reality.
Smart L, Fawkes N, Goggin P, Pennick G, Rainsford KD, Charlesworth B, Shah N. Smart L, et al. Inflammopharmacology. 2020 Oct;28(5):1141-1152. doi: 10.1007/s10787-020-00745-z. Epub 2020 Aug 14. Inflammopharmacology. 2020. PMID: 32797326 Free PMC article. Review. - Focus on Brain Angiotensin III and Aminopeptidase A in the Control of Hypertension.
Wright JW, Mizutani S, Harding JW. Wright JW, et al. Int J Hypertens. 2012;2012:124758. doi: 10.1155/2012/124758. Epub 2012 Jun 26. Int J Hypertens. 2012. PMID: 22792446 Free PMC article.
References
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme: cloning and functional expression as a captopril-insensitive carboxypeptidase. Journal of Biological Chemistry. 2000;275(43):33238–33243. - PubMed
- Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circulation Research. 2000;87(5):E1–E9. - PubMed
- Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. Journal of Biological Chemistry. 2002;277(17):14838–14843. - PubMed
- Dales NA, Gould AE, Brown JA, et al. Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors. Journal of the American Chemical Society. 2002;124(40):11852–11853. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous