A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma - PubMed (original) (raw)
Clinical Trial
doi: 10.1186/1479-5876-9-204.
Henrik Schmidt, Aviram Nissan, Laura Ridolfi, Steinar Aamdal, Johan Hansson, Michele Guida, David M Hyams, Henry Gómez, Lars Bastholt, Scott D Chasalow, David Berman
Affiliations
- PMID: 22123319
- PMCID: PMC3239318
- DOI: 10.1186/1479-5876-9-204
Clinical Trial
A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma
Omid Hamid et al. J Transl Med. 2011.
Abstract
Background: Ipilimumab, a fully human monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4, has demonstrated an improvement in overall survival in two phase III trials of patients with advanced melanoma. The primary objective of the current trial was to prospectively explore candidate biomarkers from the tumor microenvironment for associations with clinical response to ipilimumab.
Methods: In this randomized, double-blind, phase II biomarker study (ClinicalTrials.gov NCT00261365), 82 pretreated or treatment-naïve patients with unresectable stage III/IV melanoma were induced with 3 or 10 mg/kg ipilimumab every 3 weeks for 4 doses; at Week 24, patients could receive maintenance doses every 12 weeks. Efficacy was evaluated per modified World Health Organization response criteria and safety was assessed continuously. Candidate biomarkers were evaluated in tumor biopsies collected pretreatment and 24 to 72 hours after the second ipilimumab dose. Polymorphisms in immune-related genes were also evaluated.
Results: Objective response rate, response patterns, and safety were consistent with previous trials of ipilimumab in melanoma. No associations between genetic polymorphisms and clinical activity were observed. Immunohistochemistry and histology on tumor biopsies revealed significant associations between clinical activity and high baseline expression of FoxP3 (p = 0.014) and indoleamine 2,3-dioxygenase (p = 0.012), and between clinical activity and increase in tumor-infiltrating lymphocytes (TILs) between baseline and 3 weeks after start of treatment (p = 0.005). Microarray analysis of mRNA from tumor samples taken pretreatment and post-treatment demonstrated significant increases in expression of several immune-related genes, and decreases in expression of genes implicated in cancer and melanoma.
Conclusions: Baseline expression of immune-related tumor biomarkers and a post-treatment increase in TILs may be positively associated with ipilimumab clinical activity. The observed pharmacodynamic changes in gene expression warrant further analysis to determine whether treatment-emergent changes in gene expression may be associated with clinical efficacy. Further studies are required to determine the predictive value of these and other potential biomarkers associated with clinical response to ipilimumab.
Figures
Figure 1
Dosing and testing schedule: CA184-004. *Tumor biopsy was performed at baseline and 24 to 72 hours after the second dose of ipilimumab. †Influenza/pneumococcal booster administered 5 days after first dose of ipilimumab. Q3W: every 3 weeks; Q12W: every 12 weeks.
Figure 2
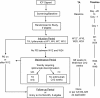
CONSORT diagram: study CA184-004. *Mandatory TA. CONSORT: Consolidated Standards of Reporting Trials; ICF: informed consent form; PD: progressive disease; TA: tumor assessment; W: study week.
Figure 3
Tumor tissue samples from patients with malignant melanoma treated with ipilimumab. 60× images of skin (A, B, F, Subject 04055; E, Subject 04024) and soft tissue (C, Subject 04066; D, Subject 04002) involved with metastasis and infiltration of melanoma cells, respectively. (A-B) Skin under the epidermis stained with hematoxylin and eosin (H&E) before (A) and after (B) treatment with ipilimumab. Melanoma cells are characterized by abundant cytoplasm, large and central nuclei, apparent nucleolus (large arrows). In contrast, melanin pigment (star) is associated with mononuclear leukocytes (small arrows). Note the increase in tumor-infiltrating mononuclear leukocytes (TILs) post-treatment (B) relative to the baseline (A) in this clinical benefit subject. (C-D) FoxP3 positive staining (with anti-FOXP3) of the nuclei of mononuclear leukocytes (small arrows) in a clinical benefit subject at baseline (D). Non-clinical benefit subject (C) shows no staining of mononuclear leukocytes at baseline. (E-F) IDO expression (anti-IDO staining) at baseline in a clinical benefit subject (F) shows staining of mononuclear leukocytes (small arrows), and spindloid and endothelial cells (large arrows). IDO expression is minimal in the non-clinical benefit subject (E) at baseline, showing focal and weak staining of melanoma cells (red arrow). H&E scores for tumor-associated infiltrating mononuclear leukocytes: (A) ≤ 50%, (B) > 50%. Staining scores for FoxP3: (C) 0, (D) 1. Staining scores for IDO: (E) 0, (F) 1.
Similar articles
- Nivolumab plus ipilimumab in advanced melanoma.
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Wolchok JD, et al. N Engl J Med. 2013 Jul 11;369(2):122-33. doi: 10.1056/NEJMoa1302369. Epub 2013 Jun 2. N Engl J Med. 2013. PMID: 23724867 Free PMC article. Clinical Trial. - Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma.
Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G, Kroeger J, Eysmans C, Sarnaik AA, Chen YA. Weber JS, et al. J Clin Oncol. 2013 Dec 1;31(34):4311-8. doi: 10.1200/JCO.2013.51.4802. Epub 2013 Oct 21. J Clin Oncol. 2013. PMID: 24145345 Free PMC article. Clinical Trial. - Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study.
Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O'Day SJ, Lebbé C. Wolchok JD, et al. Lancet Oncol. 2010 Feb;11(2):155-64. doi: 10.1016/S1470-2045(09)70334-1. Epub 2009 Dec 8. Lancet Oncol. 2010. PMID: 20004617 Clinical Trial. - Ipilimumab: a novel immunostimulatory monoclonal antibody for the treatment of cancer.
Graziani G, Tentori L, Navarra P. Graziani G, et al. Pharmacol Res. 2012 Jan;65(1):9-22. doi: 10.1016/j.phrs.2011.09.002. Epub 2011 Sep 10. Pharmacol Res. 2012. PMID: 21930211 Review. - Profile of ipilimumab and its role in the treatment of metastatic melanoma.
Patel SP, Woodman SE. Patel SP, et al. Drug Des Devel Ther. 2011;5:489-95. doi: 10.2147/DDDT.S10945. Epub 2011 Dec 16. Drug Des Devel Ther. 2011. PMID: 22267918 Free PMC article. Review.
Cited by
- Incidence of Cutaneous Immune-Related Adverse Events and Outcomes in Immune Checkpoint Inhibitor-Containing Regimens: A Systematic Review and Meta-Analysis.
Curkovic NB, Bai K, Ye F, Johnson DB. Curkovic NB, et al. Cancers (Basel). 2024 Jan 13;16(2):340. doi: 10.3390/cancers16020340. Cancers (Basel). 2024. PMID: 38254829 Free PMC article. Review. - Objective measurement and clinical significance of TILs in non-small cell lung cancer.
Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, Herbst RS, Rimm DL. Schalper KA, et al. J Natl Cancer Inst. 2015 Feb 3;107(3):dju435. doi: 10.1093/jnci/dju435. Print 2015 Mar. J Natl Cancer Inst. 2015. PMID: 25650315 Free PMC article. - Checkpoint blockade-based immunotherapy in the context of tumor microenvironment: Opportunities and challenges.
Duan J, Wang Y, Jiao S. Duan J, et al. Cancer Med. 2018 Sep;7(9):4517-4529. doi: 10.1002/cam4.1722. Epub 2018 Aug 7. Cancer Med. 2018. PMID: 30088347 Free PMC article. Review. - Clinical experience with ipilimumab 10 mg/kg in patients with melanoma treated at Italian centres as part of a European expanded access programme.
Altomonte M, Di Giacomo A, Queirolo P, Ascierto P, Spagnolo F, Bajetta E, Calabrò L, Danielli R, de Rosa F, Maur M, Chiarion-Sileni V, Ferrucci P, Giannarelli D, Testori A, Ridolfi R, Maio M. Altomonte M, et al. J Exp Clin Cancer Res. 2013 Oct 25;32(1):82. doi: 10.1186/1756-9966-32-82. J Exp Clin Cancer Res. 2013. PMID: 24423086 Free PMC article. - Expression profiles of immune-related genes are associated with neoadjuvant ipilimumab clinical benefit.
Tarhini AA, Lin Y, Lin HM, Vallabhaneni P, Sander C, LaFramboise W, Hamieh L. Tarhini AA, et al. Oncoimmunology. 2016 Sep 19;6(2):e1231291. doi: 10.1080/2162402X.2016.1231291. eCollection 2017. Oncoimmunology. 2016. PMID: 28344862 Free PMC article.
References
- Korn EL, Liu P-Y, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA, Parulekar W, Markovic SN, Saxman S, Kirkwood JM. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin OncoI. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. - DOI - PubMed
- Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. - DOI - PubMed
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical