Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fiber level? - PubMed (original) (raw)
Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fiber level?
Rizwan Qaisar et al. FASEB J. 2012 Mar.
Abstract
Muscle force is typically proportional to muscle size, resulting in constant force normalized to muscle fiber cross-sectional area (specific force). Mice overexpressing insulin-like growth factor-1 (IGF-1) exhibit a proportional gain in muscle force and size, but not the myostatin-deficient mice. In an attempt to explore the role of the cytoplasmic volume supported by individual myonuclei [myonuclear domain (MND) size] on functional capacity of skeletal muscle, we have investigated specific force in relation to MND and the content of the molecular motor protein, myosin, at the single muscle fiber level from myostatin-knockout (Mstn(-/-)) and IGF-1-overexpressing (mIgf1(+/+)) mice. We hypothesize that the addition of extra myonuclei is a prerequisite for maintenance of specific force during muscle hypertrophy. A novel algorithm was used to measure individual MNDs in 3 dimensions along the length of single muscle fibers from the fast-twitch extensor digitorum longus and the slow-twitch soleus muscle. A significant effect of the size of individual MNDs in hypertrophic muscle fibers on both specific force and myosin content was observed. This effect was muscle cell type specific and suggested there is a critical volume individual myonuclei can support efficiently. The large MNDs found in fast muscles of Mstn(-/-) mice were correlated with the decrement in specific force and myosin content in Mstn(-/-) muscles. Thus, myostatin inhibition may not be able to maintain the appropriate MND for optimal function.
Figures
Figure 1.
Confocal images of single muscle fibers and representative images of myonuclei shape in control (A, D), _Mstn_−/− (B, E) and IGF-1-overexpressing mice (C, F) in EDL (A–C) and soleus (D–F) fibers. DAPI (blue) stained myonuclei and rhodamine phalloidin (red)-labeled actin. Scale bars = 50 μm (fiber); 3 μm (nuclei).
Figure 2.
Box plots show
sd
of MND sizes from individual muscle fiber segments from EDL (A) and soleus (B) muscles. Boxes represent 25th and 75th percentiles; median value is indicated as a band near the middle of the box. Horizontal bars denote the 10th and 90th percentiles; data outside this range are indicated as dots.
Figure 3.
Enzyme histochemical myofibrillar ATPase, pH 4.5, (A–C) and SDH (D–F) staining of soleus muscle cross-sections from control (A, D), _Mstn_−/− (B, E), and mIgf1+/+ (C, F) mice, respectively. Muscle fiber types I and IIa are shown. Scale bar = 50 μm.
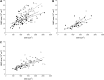
Figure 4.
Average single muscle fiber MND vs. fiber CSA in single EDL (A) and type I soleus (B) and type IIa soleus (C) muscle fibers from control (solid circles, solid line), _Mstn_−/− (open circles, dashed line), and mIgf1+/+ (open triangles, dotted line) mice.
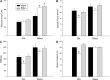
Figure 5.
Single muscle fiber absolute force (A), specific force (B), and stiffness (C) together with myosin content, described as percentage (D). Results from single muscle fibers expressing the type IIb MyHC isoform in the EDL and the IIa MyHC isoform in the soleus are included. Values are means ±
se.
*P < 0.05 vs. control; 1-way ANOVA.
Similar articles
- Decreased specific force and power production of muscle fibers from myostatin-deficient mice are associated with a suppression of protein degradation.
Mendias CL, Kayupov E, Bradley JR, Brooks SV, Claflin DR. Mendias CL, et al. J Appl Physiol (1985). 2011 Jul;111(1):185-91. doi: 10.1152/japplphysiol.00126.2011. Epub 2011 May 12. J Appl Physiol (1985). 2011. PMID: 21565991 Free PMC article. - Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle.
Girgenrath S, Song K, Whittemore LA. Girgenrath S, et al. Muscle Nerve. 2005 Jan;31(1):34-40. doi: 10.1002/mus.20175. Muscle Nerve. 2005. PMID: 15468312 - Perlecan deficiency causes muscle hypertrophy, a decrease in myostatin expression, and changes in muscle fiber composition.
Xu Z, Ichikawa N, Kosaki K, Yamada Y, Sasaki T, Sakai LY, Kurosawa H, Hattori N, Arikawa-Hirasawa E. Xu Z, et al. Matrix Biol. 2010 Jul;29(6):461-70. doi: 10.1016/j.matbio.2010.06.001. Epub 2010 Jun 9. Matrix Biol. 2010. PMID: 20541011 Free PMC article. - Gravitational unloading effects on muscle fiber size, phenotype and myonuclear number.
Ohira Y, Yoshinaga T, Nomura T, Kawano F, Ishihara A, Nonaka I, Roy RR, Edgerton VR. Ohira Y, et al. Adv Space Res. 2002;30(4):777-81. doi: 10.1016/s0273-1177(02)00395-2. Adv Space Res. 2002. PMID: 12530363 Review. - Muscle mechanics: adaptations with exercise-training.
Fitts RH, Widrick JJ. Fitts RH, et al. Exerc Sport Sci Rev. 1996;24:427-73. Exerc Sport Sci Rev. 1996. PMID: 8744258 Review.
Cited by
- Aberrant myonuclear domains and impaired myofiber contractility despite marked hypertrophy in MYMK-related, Carey-Fineman-Ziter Syndrome.
Dugdale HF, Levy Y, Jungbluth H, Oldfors A, Ochala J. Dugdale HF, et al. Acta Neuropathol Commun. 2024 May 24;12(1):80. doi: 10.1186/s40478-024-01783-2. Acta Neuropathol Commun. 2024. PMID: 38790073 Free PMC article. - The myonuclear domain in adult skeletal muscle fibres: past, present and future.
Bagley JR, Denes LT, McCarthy JJ, Wang ET, Murach KA. Bagley JR, et al. J Physiol. 2023 Feb;601(4):723-741. doi: 10.1113/JP283658. Epub 2023 Jan 30. J Physiol. 2023. PMID: 36629254 Free PMC article. Review. - Sarcopenia in pulmonary diseases is associated with elevated sarcoplasmic reticulum stress and myonuclear disorganization.
Qaisar R, Ustrana S, Muhammad T, Shah I. Qaisar R, et al. Histochem Cell Biol. 2022 Jan;157(1):93-105. doi: 10.1007/s00418-021-02043-3. Epub 2021 Oct 19. Histochem Cell Biol. 2022. PMID: 34665327 - Is the myonuclear domain ceiling hypothesis dead?
Aman F, El Khatib E, AlNeaimi A, Mohamed A, Almulla AS, Zaidan A, Alshafei J, Habbal O, Eldesouki S, Qaisar R. Aman F, et al. Singapore Med J. 2023 Jul;64(7):415-422. doi: 10.11622/smedj.2021103. Singapore Med J. 2023. PMID: 34544215 Free PMC article. Review. - Antimyostatin Treatment in Health and Disease: The Story of Great Expectations and Limited Success.
Nielsen TL, Vissing J, Krag TO. Nielsen TL, et al. Cells. 2021 Mar 3;10(3):533. doi: 10.3390/cells10030533. Cells. 2021. PMID: 33802348 Free PMC article. Review.
References
- Allen D. L., Monke S. R., Talmadge R. J., Roy R. R., Edgerton V. R. (1995) Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J. Appl. Physiol. 78, 1969–1976 - PubMed
- Allen R. E., Boxhorn L. K. (1989) Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J. Cell. Physiol. 138, 311–315 - PubMed
- Dodson M. V., Allen R. E., Hossner K. L. (1985) Ovine somatomedin, multiplication-stimulating activity, and insulin promote skeletal muscle satellite cell proliferation in vitro. Endocrinology 117, 2357–2363 - PubMed
- Barton-Davis E. R., Shoturma D. I., Sweeney H. L. (1999) Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol. Scand. 167, 301–305 - PubMed
- McCarthy J. J., Esser K. A. (2007) Satellite cell addition is not obligatory for skeletal muscle hypertrophy. J Appl. Physiol. 103, 1100–1102; discussion 1102–1103 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous