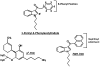1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice - PubMed (original) (raw)
1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice
Jenny L Wiley et al. Drug Alcohol Depend. 2012.
Abstract
Background: Smoking of synthetic cannabinoid-enhanced "herbal incense" is an emerging substance abuse problem. The indole-derived cannabinoids identified in these products were originally developed as research tools and are structurally distinct from cannabinoids in the cannabis plant. Although abused by humans, most published research on this class of compounds has been performed in vitro. The purpose of this study was to evaluate a novel series of 1-pentyl-3-phenylacetylindoles in mice.
Methods: The potencies of these analogs to produce the cannabinoid agonist effects of antinociception, hypothermia and suppression of locomotion were evaluated in ICR mice. The major structural manipulations in the present series included the type of substituent (i.e., unsubstituted, methyl, methoxy, chloro, bromo, and fluoro) and the position of the substituent on the phenyl ring (i.e., 2-, 3- or 4-position).
Results: Potencies of this series of phenylacetylindoles for each cannabinoid effect were highly correlated with CB(1) receptor affinities reported previously. Active compounds produced a profile of effects that resembled that exhibited by Δ(9)-tetrahydrocannabinol (THC). The most critical factor affecting in vivo potency was the position of the substituent. Whereas compounds with 2- and 3-phenylacetyl substituents were efficacious with good potencies, 4-substituents resulted in compounds that had poor potency or were inactive.
Conclusions: These results suggest that phenylacetylindoles with good CB(1) binding affinity share pharmacological properties with THC in mice; however, they also emphasize the complexity of molecular interactions of synthetic cannabinoids with CB(1) receptors and suggest that scheduling efforts based solely upon structural features should proceed with caution.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
5.3 Conflicts of interst
All of the authors declare that they have no conflicts of interest.
Figures
Figure 1
Chemical structures of THC and JWH-018. The structural template for the 1-pentyl-3-phenylacetylindoles is also shown, with positions of R and R′ groups indicated (see Tables 1 and 2).
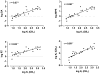
Figure 2
Scatterplots and regression lines for CB1 affininities (log Ki) plotted against log ED50 for each of the three in vivo tests (SA = spontaneous activity, top left panel; MPE = % maximum possible antinociceptive effect, top right panel; RT = change in rectal temperature, bottom left panel). The bottom right panel shows scatterplot and regression line for the relationship between CB1 and CB2 binding affinities (log Kis). Pearson product-moment correlations are shown for the two measures graphed in each panel. * indicates significant correlation (p<0.05).
Similar articles
- Thermolytic Degradation of Synthetic Cannabinoids: Chemical Exposures and Pharmacological Consequences.
Thomas BF, Lefever TW, Cortes RA, Grabenauer M, Kovach AL, Cox AO, Patel PR, Pollard GT, Marusich JA, Kevin RC, Gamage TF, Wiley JL. Thomas BF, et al. J Pharmacol Exp Ther. 2017 Apr;361(1):162-171. doi: 10.1124/jpet.116.238717. Epub 2017 Jan 13. J Pharmacol Exp Ther. 2017. PMID: 28087785 Free PMC article. - 3-Substituted pyrazole analogs of the cannabinoid type 1 (CB₁) receptor antagonist rimonabant: cannabinoid agonist-like effects in mice via non-CB₁, non-CB₂ mechanism.
Wiley JL, Selley DE, Wang P, Kottani R, Gadthula S, Mahadeven A. Wiley JL, et al. J Pharmacol Exp Ther. 2012 Feb;340(2):433-44. doi: 10.1124/jpet.111.187815. Epub 2011 Nov 15. J Pharmacol Exp Ther. 2012. PMID: 22085649 Free PMC article. - AB-CHMINACA, AB-PINACA, and FUBIMINA: Affinity and Potency of Novel Synthetic Cannabinoids in Producing Δ9-Tetrahydrocannabinol-Like Effects in Mice.
Wiley JL, Marusich JA, Lefever TW, Antonazzo KR, Wallgren MT, Cortes RA, Patel PR, Grabenauer M, Moore KN, Thomas BF. Wiley JL, et al. J Pharmacol Exp Ther. 2015 Sep;354(3):328-39. doi: 10.1124/jpet.115.225326. Epub 2015 Jun 23. J Pharmacol Exp Ther. 2015. PMID: 26105953 Free PMC article. - Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids.
Wiley JL, Marusich JA, Huffman JW. Wiley JL, et al. Life Sci. 2014 Feb 27;97(1):55-63. doi: 10.1016/j.lfs.2013.09.011. Epub 2013 Sep 23. Life Sci. 2014. PMID: 24071522 Free PMC article. Review. - Combination Chemistry: Structure-Activity Relationships of Novel Psychoactive Cannabinoids.
Wiley JL, Marusich JA, Thomas BF. Wiley JL, et al. Curr Top Behav Neurosci. 2017;32:231-248. doi: 10.1007/7854_2016_17. Curr Top Behav Neurosci. 2017. PMID: 27753007 Review.
Cited by
- The synthesis and pharmacological evaluation of adamantane-derived indoles: cannabimimetic drugs of abuse.
Banister SD, Wilkinson SM, Longworth M, Stuart J, Apetz N, English K, Brooker L, Goebel C, Hibbs DE, Glass M, Connor M, McGregor IS, Kassiou M. Banister SD, et al. ACS Chem Neurosci. 2013 Jul 17;4(7):1081-92. doi: 10.1021/cn400035r. Epub 2013 Apr 17. ACS Chem Neurosci. 2013. PMID: 23551277 Free PMC article. - The Directive 2010/63/EU on animal experimentation may skew the conclusions of pharmacological and behavioural studies.
Macrì S, Ceci C, Altabella L, Canese R, Laviola G. Macrì S, et al. Sci Rep. 2013;3:2380. doi: 10.1038/srep02380. Sci Rep. 2013. PMID: 23924859 Free PMC article. - Potential Mechanisms Underlying the Deleterious Effects of Synthetic Cannabinoids Found in Spice/K2 Products.
Basavarajappa BS, Subbanna S. Basavarajappa BS, et al. Brain Sci. 2019 Jan 16;9(1):14. doi: 10.3390/brainsci9010014. Brain Sci. 2019. PMID: 30654473 Free PMC article. Review. - Neurotoxicity of Synthetic Cannabinoids JWH-081 and JWH-210.
Cha HJ, Seong YH, Song MJ, Jeong HS, Shin J, Yun J, Han K, Kim YH, Kang H, Kim HS. Cha HJ, et al. Biomol Ther (Seoul). 2015 Nov;23(6):597-603. doi: 10.4062/biomolther.2015.057. Epub 2015 Nov 1. Biomol Ther (Seoul). 2015. PMID: 26535086 Free PMC article. - Neurocognition and subjective experience following acute doses of the synthetic cannabinoid JWH-018: a phase 1, placebo-controlled, pilot study.
Theunissen EL, Hutten NRPW, Mason NL, Toennes SW, Kuypers KPC, de Sousa Fernandes Perna EB, Ramaekers JG. Theunissen EL, et al. Br J Pharmacol. 2018 Jan;175(1):18-28. doi: 10.1111/bph.14066. Epub 2017 Nov 29. Br J Pharmacol. 2018. PMID: 29164599 Free PMC article. Clinical Trial.
References
- Balster RL, Prescott WR. Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. - PubMed
- Barrett RL, Wiley JL, Balster RL, Martin BR. Pharmacological specificity of delta-9-tetrahydrocannabinol discrimination in rats. Psychopharmacology (Berl) 1995;118:419–424. - PubMed
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta-9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992a;263:1118–1126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA003590-21/DA/NIDA NIH HHS/United States
- R01 DA003672/DA/NIDA NIH HHS/United States
- DA-03590/DA/NIDA NIH HHS/United States
- DA-03672/DA/NIDA NIH HHS/United States
- DA-031988/DA/NIDA NIH HHS/United States
- R03 DA031988/DA/NIDA NIH HHS/United States
- R03 DA031988-01/DA/NIDA NIH HHS/United States
- R37 DA003672/DA/NIDA NIH HHS/United States
- R01 DA003590/DA/NIDA NIH HHS/United States
- R01 DA003672-29/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources