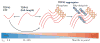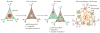Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration - PubMed (original) (raw)
Review
Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration
Edward B Lee et al. Nat Rev Neurosci. 2011.
Abstract
RNA-binding proteins, and in particular TAR DNA-binding protein 43 (TDP43), are central to the pathogenesis of motor neuron diseases and related neurodegenerative disorders. Studies on human tissue have implicated several possible mechanisms of disease and experimental studies are now attempting to determine whether TDP43-mediated neurodegeneration results from a gain or a loss of function of the protein. In addition, the distinct possibility of pleotropic or combined effects - in which gains of toxic properties and losses of normal TDP43 functions act together - needs to be considered.
Conflict of interest statement
Competing interests statement
The authors declare no competing financial interests.
Figures
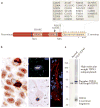
Figure 1. The genetics, pathology and biochemistry of TDP43 proteinopathies
a | TAR DNA-binding protein 43 (TDP43) protein contains two RNA-recognition motifs (RNA-recognition motif 1 (RRM1) and RRM2), a carboxy-terminal glycine-rich domain, a bipartite nuclear localization signal (NLS) and a nuclear export signal (NES). Numerous mutations (shown by short vertical lines) have been linked to sporadic and familial forms of amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). These are almost exclusively found within or immediately adjacent to the glycine-rich domain with the exception of an Asp169Gly mutation within exon 4 (the site at which TDP43 cleavage putatively occurs is shown by an arrow). Known TDP43 phosphorylation sites (Ser 379, Ser403 + Ser404, and Ser409 + Ser 410), when heavily phosphorylated, also contribute to the disease-specific TDP43 biochemical signature (these sites are indicated by asterisks). b | TDP43 immunohistochemistry of human FTLD brain reveals intracytoplasmic inclusions affecting neurons. Dystrophic neurites and glia also exhibit TDP43 inclusions (not shown). An image of the dentate gyrus stained with an anti-TDP43 antibody shows cells without inclusions with normal nuclear immunoreactivity, whereas inclusion-bearing cells show a loss of normal nuclear staining that leads to a presumptive loss of TDP43 function in the nucleus. TDP43 immunofluorescence (shown in green) of an intranuclear inclusion in FTLD-TDP43 is shown. TDP43 immunostaining using a phospho-specific anti-TDP43 antibody of human ALS spinal cord shows characteristic round inclusions (shown by brown immunohistochemistry) and skeins (shown by red immunofluorescence) in lower motor neurons. c | Biochemical analysis demonstrates the distinct biochemical disease-specific TDP43 signature characterized by the accumulation of sarkosyl-insoluble TDP43 species comprised of phosphorylated TDP43, ubiquitylated high molecular weight TDP43 and truncated C-terminal fragments.
Figure 2. Normal functions of TDP43
TAR DNA-binding protein 43 (TDP43) exhibits multiple normal biological functions, predominantly those that regulate RNA pathways. a | TDP43 is a component of heterogenous nuclear ribonucleoprotein (hnRNP) particles, which regulate splicing of pre-mRNA species. b | TDP43 also binds to mRNA sequences, particularly within the 3′ untranslated region, and affects mRNA stability and turnover. c | TDP43 is thought to play a part in mRNA trafficking, as TDP43 undergoes rapid nucleo–cytoplasmic shuttling and is localized within dendritic RNA granules. d | TDP43 is also a component of the Drosha complex, which functions to process primary microRNAs. e | TDP43 can act as a transcriptional repressor by binding to single stranded DNA (ssDNA) promoter sequences. f | TDP43 also colocalizes with stress granules that are thought to sequester and protect mRNAs under conditions of stress.
Figure 3. TDP43 modification, stability and turnover
Both proteosomal and autophagasomal degradation of TAR DNA-binding protein 43 (TDP43) protein has been described. We found that full-length TDP43 is a long-lived protein with a half-life of greater than 34 hours, although other studies have reported that it has a half-life ranging from 4 to 12 hours depending on the cell type,. Truncation of TDP43 results in the production of carboxy-terminal fragments (CTFs) that are rapidly translocated to the cytoplasm and degraded. TDP43 aggregates can form under various conditions in which CTFs and full-length TDP43 protein seem to co-aggregate, and TDP43 aggregation is tightly linked with the presence of phosphorylated TDP43. Ubiquitylation of TDP43 aggregates seems to occur late in the lifecycle of an inclusion. Given that TDP43 aggregates are resistant to degradation, different TDP43 isoforms and conformers exhibit different turnover rates, ranging from the labile soluble CTFs to stable insoluble aggregates. t1/2, half-life.
Figure 4. Lifecycle of TDP43 pathology
Normal neurons show robust intranuclear TAR DNA-binding protein 43 (TDP43) immunoreactivity (shown in red) with little cytoplasmic TDP43. So-called ‘pre-inclusions’ have been described, and these consist of granular cytoplasmic aggregates that are positive for phospho-TDP43 epitopes (p409 and p410) but that are often negative for ubiquitin. Neurons with pre-inclusions show characteristic loss of normal nuclear staining. Bona fide inclusions exhibit a variety of morphologies ranging from dense round inclusions and skeins in motor neurons to dystrophic neurites, cytoplasmic inclusions or intranuclear ‘cat eye’ inclusions in other neurons. Neuronophagia can rarely be seen in amyotrophic lateral sclerosis, and neuronophagic cell clusters have been reported to be associated with TDP43 inclusions.
Figure 5. Subcellular localization of TDP43 protein
TAR DNA-binding protein 43 (TDP43) shows a predominantly nuclear localization, although nucleo–cytoplasmic shuttling of TDP43 has been shown and low levels of cytoplasmic TDP43 can be demonstrated. TDP43 accumulation in the cytoplasm can be induced by a variety of cellular stressors that result in the formation of TDP43-positive stress granules. TDP43 protein has also been found in RNA transport granules within neuronal processes. TDP43 protein is thought to undergo proteolytic cleavage by caspase 3 to generate a carboxy-terminal fragment (CTF). As the CTF no longer contains the bipartite nuclear localization signal (NLS, shown by a red rectangle), CTFs translocate into the cytoplasm, where they may participate in aggregate formation. Experimentally generated mutations of the NLS and disease-associated mutations (shown by a yellow lightning bolt) have been found to increase the amount of cytoplasmic versus nuclear TDP43. MG132 is a proteasome inhibitor.
Figure 6. Autoregulation of TDP43 and models of TDP43 toxicity
a | Normal TAR DNA-binding protein 43 (TDP43) autoregulation. TDP43 expression is tightly regulated under normal conditions, and overexpression of exogenous TDP43 results in a reduction of endogenous TDP43 expression. This autoregulation of TDP43 expression by itself is mediated at the level of mRNA stability. Within the 3′ untranslated region (UTR) of TDP43 mRNA is a binding site for TDP43 protein that is critical for autoregulation. TDP43 binding to this 3′ UTR site promotes degradation of TDP43 mRNA, at least partly through the exosome. There are conflicting reports about the mechanism of autoregulation, in particular whether alternative splicing leading to nonsense-mediated decay (NMD) is the mechanism of TDP43 autoregulation. b | Loss of autoregulation. According to this model, the exposure of neurons to as-yet-unidentified stressors can lead to cytoplasmic mislocalization of TDP43 (1), and this is perhaps related to the stress granule response. Given TDP43’s propensity to aggregate, TDP43 forms phosphorylated pre-inclusions within the cytoplasm that sequester free TDP43 protein. This cytoplasmic sequestration leads to a loss of normal nuclear TDP43. If autoregulation occurs within the nucleus, a loss of TDP43 autoregulation ensues (2), which results in increased TDP43 mRNA and protein (3). This further exacerbates TDP43 aggregation (4). This vicious cycle leads to cell death (5) possibly through a variety of gain and loss of functions. c | Gain of autoregulation. In this model, neurons that are exposed to unknown stressors can undergo cytoplasmic mislocalization of TDP43 protein (1), and this is perhaps related to the stress granule response. Given the propensity of TDP43 to aggregate, TDP43 forms phosphorylated pre-inclusions within the cytoplasm which are resistant to degradation. If autoregulation occurs within the cytoplasm, an increase in cytoplasmic TDP43 may result in an increase in TDP43 autoregulation (2) that would decrease TDP43 mRNA and therefore decrease synthesis of new TDP43 protein (3). This reduction in TDP43 protein synthesis leads to a loss of normal nuclear TDP43 protein. Given the plethora of normal nuclear TDP43 functions, the absence of nuclear TDP43 is detrimental to neuronal viability, increasing the stress response (4) and leading to cell death (5). Again, this model allows for the possibly of a variety of gain and loss of functions that coordinately result in toxicity.
Similar articles
- Age-dependent neurodegeneration and organelle transport deficiencies in mutant TDP43 patient-derived neurons are independent of TDP43 aggregation.
Kreiter N, Pal A, Lojewski X, Corcia P, Naujock M, Reinhardt P, Sterneckert J, Petri S, Wegner F, Storch A, Hermann A. Kreiter N, et al. Neurobiol Dis. 2018 Jul;115:167-181. doi: 10.1016/j.nbd.2018.03.010. Epub 2018 Apr 6. Neurobiol Dis. 2018. PMID: 29630989 - An Intramolecular Salt Bridge Linking TDP43 RNA Binding, Protein Stability, and TDP43-Dependent Neurodegeneration.
Flores BN, Li X, Malik AM, Martinez J, Beg AA, Barmada SJ. Flores BN, et al. Cell Rep. 2019 Apr 23;27(4):1133-1150.e8. doi: 10.1016/j.celrep.2019.03.093. Cell Rep. 2019. PMID: 31018129 Free PMC article. - TDP-43-mediated neurodegeneration: towards a loss-of-function hypothesis?
Vanden Broeck L, Callaerts P, Dermaut B. Vanden Broeck L, et al. Trends Mol Med. 2014 Feb;20(2):66-71. doi: 10.1016/j.molmed.2013.11.003. Epub 2013 Dec 16. Trends Mol Med. 2014. PMID: 24355761 - TDP43 ribonucleoprotein granules: physiologic function to pathologic aggregates.
Corbet GA, Wheeler JR, Parker R, Weskamp K. Corbet GA, et al. RNA Biol. 2021 Oct 15;18(sup1):128-138. doi: 10.1080/15476286.2021.1963099. Epub 2021 Aug 19. RNA Biol. 2021. PMID: 34412568 Free PMC article. Review. - RNA-binding proteins in neurodegeneration: mechanisms in aggregate.
Conlon EG, Manley JL. Conlon EG, et al. Genes Dev. 2017 Aug 1;31(15):1509-1528. doi: 10.1101/gad.304055.117. Genes Dev. 2017. PMID: 28912172 Free PMC article. Review.
Cited by
- Neurodegeneration-associated TDP-43 interacts with fragile X mental retardation protein (FMRP)/Staufen (STAU1) and regulates SIRT1 expression in neuronal cells.
Yu Z, Fan D, Gui B, Shi L, Xuan C, Shan L, Wang Q, Shang Y, Wang Y. Yu Z, et al. J Biol Chem. 2012 Jun 29;287(27):22560-72. doi: 10.1074/jbc.M112.357582. Epub 2012 May 14. J Biol Chem. 2012. PMID: 22584570 Free PMC article. - Quantification of the Relative Contributions of Loss-of-function and Gain-of-function Mechanisms in TAR DNA-binding Protein 43 (TDP-43) Proteinopathies.
Cascella R, Capitini C, Fani G, Dobson CM, Cecchi C, Chiti F. Cascella R, et al. J Biol Chem. 2016 Sep 9;291(37):19437-48. doi: 10.1074/jbc.M116.737726. Epub 2016 Jul 21. J Biol Chem. 2016. PMID: 27445339 Free PMC article. - Pathological TDP-43 changes in Betz cells differ from those in bulbar and spinal α-motoneurons in sporadic amyotrophic lateral sclerosis.
Braak H, Ludolph AC, Neumann M, Ravits J, Del Tredici K. Braak H, et al. Acta Neuropathol. 2017 Jan;133(1):79-90. doi: 10.1007/s00401-016-1633-2. Epub 2016 Oct 18. Acta Neuropathol. 2017. PMID: 27757524 Free PMC article. - Distribution of ubiquilin 2 and TDP-43 aggregates throughout the CNS in UBQLN2 p.T487I-linked amyotrophic lateral sclerosis and frontotemporal dementia.
Nementzik LR, Thumbadoo KM, Murray HC, Gordon D, Yang S, Blair IP, Turner C, Faull RLM, Curtis MA, McLean C, Nicholson GA, Swanson MEV, Scotter EL. Nementzik LR, et al. Brain Pathol. 2024 May;34(3):e13230. doi: 10.1111/bpa.13230. Epub 2023 Dec 19. Brain Pathol. 2024. PMID: 38115557 Free PMC article. - Partial Failure of Proteostasis Systems Counteracting TDP-43 Aggregates in Neurodegenerative Diseases.
Cascella R, Fani G, Bigi A, Chiti F, Cecchi C. Cascella R, et al. Int J Mol Sci. 2019 Jul 27;20(15):3685. doi: 10.3390/ijms20153685. Int J Mol Sci. 2019. PMID: 31357627 Free PMC article.
References
- Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. TDP43 protein is identified biochemically, immunohistochemically and by amino acid sequence analysis as the major component of proteinaceous ubiquitin-positive inclusions in FTLD and ALS. Pathologic TDP43 is found to be ubiquitylated, phosphorylated and cleaved, and is associated with nuclear clearance of normal TDP43. - PubMed
- Brandmeir NJ, et al. Severe subcortical TDP-43 pathology in sporadic frontotemporal lobar degeneration with motor neuron disease. Acta Neuropathol. 2008;115:123–131. - PubMed
- Strong MJ, et al. TDP43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol Cell Neurosci. 2007;35:320–327. - PubMed
- Pamphlett R, Luquin N, McLean C, Jew SK, Adams L. TDP-43 neuropathology is similar in sporadic amyotrophic lateral sclerosis with or without TDP-43 mutations. Neuropathol Appl Neurobiol. 2009;35:222–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG10124/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- T32 AG000255/AG/NIA NIH HHS/United States
- K08 AG039510/AG/NIA NIH HHS/United States
- AG17586/AG/NIA NIH HHS/United States
- T32 AG00255/AG/NIA NIH HHS/United States
- K08AG039510/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P30 AG010124-22/AG/NIA NIH HHS/United States
- P01 AG017586-01/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases