Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis, and impaired insulin signaling in rat L6 skeletal muscle cells - PubMed (original) (raw)
Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis, and impaired insulin signaling in rat L6 skeletal muscle cells
Larysa V Yuzefovych et al. Endocrinology. 2012 Jan.
Abstract
Saturated free fatty acids have been implicated in the increase of oxidative stress, mitochondrial dysfunction, apoptosis, and insulin resistance seen in type 2 diabetes. The purpose of this study was to determine whether palmitate-induced mitochondrial DNA (mtDNA) damage contributed to increased oxidative stress, mitochondrial dysfunction, apoptosis, impaired insulin signaling, and reduced glucose uptake in skeletal muscle cells. Adenoviral vectors were used to deliver the DNA repair enzyme human 8-oxoguanine DNA glycosylase/(apurinic/apyrimidinic) lyase (hOGG1) to mitochondria in L6 myotubes. After palmitate exposure, we evaluated mtDNA damage, mitochondrial function, production of mitochondrial reactive oxygen species, apoptosis, insulin signaling pathways, and glucose uptake. Protection of mtDNA from palmitate-induced damage by overexpression of hOGG1 targeted to mitochondria significantly diminished palmitate-induced mitochondrial superoxide production, restored the decline in ATP levels, reduced activation of c-Jun N-terminal kinase (JNK) kinase, prevented cells from entering apoptosis, increased insulin-stimulated phosphorylation of serine-threonine kinase (Akt) (Ser473) and tyrosine phosphorylation of insulin receptor substrate-1, and thereby enhanced glucose transporter 4 translocation to plasma membrane, and restored insulin signaling. Addition of a specific inhibitor of JNK mimicked the effect of mitochondrial overexpression of hOGG1 and partially restored insulin sensitivity, thus confirming the involvement of mtDNA damage and subsequent increase of oxidative stress and JNK activation in insulin signaling in L6 myotubes. Our results are the first to report that mtDNA damage is the proximal cause in palmitate-induced mitochondrial dysfunction and impaired insulin signaling and provide strong evidence that targeting DNA repair enzymes into mitochondria in skeletal muscles could be a potential therapeutic treatment for insulin resistance.
Figures
Fig. 1.
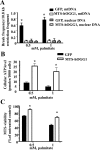
Targeting of hOGG1 to mitochondria from L6 myotubes. A, Adenoviral vectors (GFP or MTS-hOGG1) were added to cells in differentiation media at the indicated MOI. After a 48-h transduction, mitochondrial fractions were isolated from the transduced L6 myotubes, and Western blot analysis was performed using hOGG1 antiserum. Immunodetection of cytochrome c was performed to assure mitochondrial localization. B, Nuclear and cytosolic fractions were isolated from L6 myotubes transduced with GFP or MTS-hOGG1 viruses at a MOI of 70. Equal loading was confirmed using Ponceau staining of the membrane. Lamin A and actin were used to indicate nuclear and cytosolic localization, respectively. To exclude possible contamination from mitochondrial fractions, a separate portion of the same blot was probed with cytochrome c.
Fig. 2.
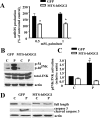
Overexpression of hOGG1 in mitochondria from L6 myotubes prevents palmitate-induced mtDNA damage and increased mitochondrial function. A, Break frequency per 10.8-kb fragment of nuclear and mtDNA after 6 h of treatment with the indicated concentrations of palmitate (n ≥ 3). *, P < 0.05 vs. all other groups. B, ATP levels were increased in L6 myotubes transduced with MTS-hOGG1 adenoviruses after palmitate treatment. Cells were transduced with the adenoviruses for 48 h and then treated with the indicated concentration of palmitate, and ATP production was measured. The mean results ±
se
are shown (n ≥ 3). *, P < 0.05 vs. GFP-transduced cells treated with the same concentration of palmitate. C, Mitochondrial function is increased in MTS-hOGG1-expressing myotubes 24 h after exposure to indicated concentrations of palmitate. The average results ±
se
are shown (n = 3). *, P < 0.05 vs. GFP-transduced cells treated with the same concentration of palmitate.
Fig. 3.
Targeting of hOGG1 to mitochondria in L6 myotubes protected against palmitate-induced mtROS generation, reduced activation of JNK kinase, and prevented cells from undergoing apoptosis. A, Mitochondrial superoxide production in MTS-hOGG1- and GFP-transduced L6 myotubes treated with the indicated concentrations of palmitate for 24 h. Cells were analyzed in a fluorescent plate reader, and the increase in ROS production was calculated as a percentage increase compared with control. The mean results ±
se
are shown (n ≥ 3). *, P < 0.05 vs. GFP-transduced cells treated with the same concentration of palmitate. B, Adenovirus-transduced L6 myotubes were exposed to control medium (C) (2% BSA) or medium containing 1 m
m
palmitate (P). Total cell lysates were isolated and analyzed by Western blotting with the indicated antibodies. Equal loading was confirmed using antiactin antibody. C, The values from densitometry from three (pJNK) independent experiments were normalized to the level of total JNK and expressed as fold of difference normalized to GFP control data ±
se
; *, P < 0.05 vs. all other groups. D, Results of the Western blottings using caspase-3 antibodies which recognize the full-length (35-kD) and the large (17 kD) fragment of caspase-3 resulting from its cleavage. Equal loading was confirmed by loading antiactin antibody.
Fig. 4.
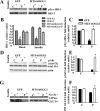
Targeting of hOGG1 to mitochondria in L6 myotubes ameliorated palmitate-mediated inhibition of insulin-induced. A, C tyrosine phosphorylation of IRS-1. D and E, Akt (Ser473) phosphorylation. G and F, GLUT4 translocation to the PM. MTS-hOGG1- or GFP-transduced L6 myotubes were exposed to control medium (C) (2% BSA) or to medium containing 1 m
m
palmitate (P) for 16 h and then treated with insulin as described in Materials and Methods. Total cell lysates or PM (as specified) were isolated and analyzed by Western blot analysis with the indicated antibodies. A, top panel, IRS-1 was immunoprecipitated from 200 μg of total cell lysates, and Western blottings were performed using an pTyr antibody. Bottom, Representative Western blotting of total IRS-1. B, Densitometry data for the total IRS-1 protein levels normalized to GFP control data (n ≥ 3). *, P < 0.05 vs. all other groups. C, Densitometry data for insulin dependent (pTyr-IRS-1) were normalized to GFP (control plus insulin) data and presented as means ±
se
(n ≥ 3). *, P < 0.05 vs. GFP-transduced cells treated with palmitate. D, Representative blots from at least three independent experiments for phosphorylation of Akt (Ser473) are shown. Total cell lysates were used. E, The values from densitometry from at least three (pAkt) independent experiments were normalized to the level of total Akt and expressed as fold of difference after addition of insulin normalized to the GFP (control plus insulin) data. The mean results ±
se
are shown. *, P < 0.05 vs. GFP-transduced cells treated with palmitate. G, PM were isolated from adenovirus-transduced L6 myotubes, as described in Materials and Methods, and proteins were analyzed by Western blotting using GLUT4 antibody. Immunodetection of the α-subunit of Na+/K+-ATPase was performed to confirm PM localization. The values from densitometry performed on three to four independent GLUT4 translocation to PM independent experiments were normalized to the level of α-subunit of Na+/K+-ATPase and then normalized to the GFP (control plus insulin) data ±
se
(n ≥ 3). *, P < 0.05 vs. GFP-transduced cells treated with palmitate.
Fig. 5.
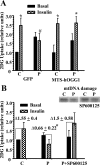
Targeting of hOGG1 to mitochondria in L6 myotubes protected against palmitate-induced decrease in insulin-stimulated 2DG uptake. A, MTS-hOGG1- and GFP-transduced L6 myotubes were treated with 2% BSA (C, 2% BSA) or 2% BSA plus 1 m
m
palmitate (P) for 16 h. After that, cells were incubated in the absence or presence of insulin for 20 min and then for 5 min with 2DG, and uptake was measured as described in Materials and Methods. Values were normalized to the GFP control basal data and are the means ±
se
(n ≥ 3). *, P < 0.05 vs. respective basal; #, P < 0.05 vs. all other groups treated with insulin. B, graph, Effect of a JNK inhibitor on palmitate-induced inhibition of insulin-stimulated 2DG uptake. L6 myotubes were incubated in the medium containing only 2% BSA (C, Control) (2% BSA) or 2% BSA plus 1 m
m
palmitate (P) in the presence or absence of the JNK inhibitor, SP-600125 for 16 h before stimulation with insulin and measurement of 2DG uptake. Values were normalized to the control basal data and are the means ±
se
(n ≥ 3). Δ, Fold induction on insulin. *, P < 0.05 vs. both control and palmitate plus SP-600125. B, panel above graph, Effect of JNK inhibitor on palmitate-induced mtDNA damage. L6 myotubes were incubated with 2% BSA (C) or 2% BSA plus 1 m
m
palmitate (P) in the presence or absence of the JNK inhibitor, SP-600125 for 6 h. mtDNA damage was evaluated as described in Materials and Methods.
Similar articles
- Alteration of mitochondrial function and insulin sensitivity in primary mouse skeletal muscle cells isolated from transgenic and knockout mice: role of ogg1.
Yuzefovych LV, Schuler AM, Chen J, Alvarez DF, Eide L, Ledoux SP, Wilson GL, Rachek LI. Yuzefovych LV, et al. Endocrinology. 2013 Aug;154(8):2640-9. doi: 10.1210/en.2013-1076. Epub 2013 Jun 7. Endocrinology. 2013. PMID: 23748360 Free PMC article. - Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress.
Yuzefovych L, Wilson G, Rachek L. Yuzefovych L, et al. Am J Physiol Endocrinol Metab. 2010 Dec;299(6):E1096-105. doi: 10.1152/ajpendo.00238.2010. Epub 2010 Sep 28. Am J Physiol Endocrinol Metab. 2010. PMID: 20876761 Free PMC article. - Protein tyrosine phosphatase 1B inhibition ameliorates palmitate-induced mitochondrial dysfunction and apoptosis in skeletal muscle cells.
Taheripak G, Bakhtiyari S, Rajabibazl M, Pasalar P, Meshkani R. Taheripak G, et al. Free Radic Biol Med. 2013 Dec;65:1435-1446. doi: 10.1016/j.freeradbiomed.2013.09.019. Epub 2013 Oct 10. Free Radic Biol Med. 2013. PMID: 24120971 - A systematic review of p53 regulation of oxidative stress in skeletal muscle.
Beyfuss K, Hood DA. Beyfuss K, et al. Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article. Review. - The impact of oxidative stress-induced mitochondrial dysfunction on diabetic microvascular complications.
Zhang Z, Huang Q, Zhao D, Lian F, Li X, Qi W. Zhang Z, et al. Front Endocrinol (Lausanne). 2023 Feb 7;14:1112363. doi: 10.3389/fendo.2023.1112363. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36824356 Free PMC article. Review.
Cited by
- Mitochondrial DNA damage via augmented oxidative stress regulates endoplasmic reticulum stress and autophagy: crosstalk, links and signaling.
Yuzefovych LV, LeDoux SP, Wilson GL, Rachek LI. Yuzefovych LV, et al. PLoS One. 2013 Dec 13;8(12):e83349. doi: 10.1371/journal.pone.0083349. eCollection 2013. PLoS One. 2013. PMID: 24349491 Free PMC article. - Mild Cognitive Impairment and Donepezil Impact Mitochondrial Respiratory Capacity in Skeletal Muscle.
Morris JK, McCoin CS, Fuller KN, John CS, Wilkins HM, Green ZD, Wang X, Sharma P, Burns JM, Vidoni ED, Mahnken JD, Shankar K, Swerdlow RH, Thyfault JP. Morris JK, et al. Function (Oxf). 2021 Sep 2;2(6):zqab045. doi: 10.1093/function/zqab045. eCollection 2021. Function (Oxf). 2021. PMID: 34661111 Free PMC article. - Roles of OGG1 in transcriptional regulation and maintenance of metabolic homeostasis.
Sampath H, Lloyd RS. Sampath H, et al. DNA Repair (Amst). 2019 Sep;81:102667. doi: 10.1016/j.dnarep.2019.102667. Epub 2019 Jul 8. DNA Repair (Amst). 2019. PMID: 31311771 Free PMC article. Review. - Differential Roles of CD36 in Regulating Muscle Insulin Response Depend on Palmitic Acid Load.
Sun J, Su Y, Chen J, Qin D, Xu Y, Chu H, Lu T, Dong J, Qin L, Li W. Sun J, et al. Biomedicines. 2023 Feb 28;11(3):729. doi: 10.3390/biomedicines11030729. Biomedicines. 2023. PMID: 36979708 Free PMC article. - Alteration of mitochondrial function and insulin sensitivity in primary mouse skeletal muscle cells isolated from transgenic and knockout mice: role of ogg1.
Yuzefovych LV, Schuler AM, Chen J, Alvarez DF, Eide L, Ledoux SP, Wilson GL, Rachek LI. Yuzefovych LV, et al. Endocrinology. 2013 Aug;154(8):2640-9. doi: 10.1210/en.2013-1076. Epub 2013 Jun 7. Endocrinology. 2013. PMID: 23748360 Free PMC article.
References
- Rachek LI, Grishko VI, Musiyenko SI, Kelley MR, LeDoux SP, Wilson GL. 2002. Conditional targeting of the DNA repair enzyme hOGG1 into mitochondria. J Biol Chem 277:44932–44937 - PubMed
- Rachek LI, Grishko VI, Ledoux SP, Wilson GL. 2006. Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Radic Biol Med 40:754–762 - PubMed
- Rachek LI, Thornley NP, Grishko VI, LeDoux SP, Wilson GL. 2006. Protection of INS-1 cells from free fatty acid-induced apoptosis by targeting hOGG1 to mitochondria. Diabetes 55:1022–1028 - PubMed
- Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer D, Schaffer SW. 2008. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol Cell Physiol 294:C413–C422 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous