Dissociation of inositol-requiring enzyme (IRE1α)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice - PubMed (original) (raw)
Dissociation of inositol-requiring enzyme (IRE1α)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice
Michael J Jurczak et al. J Biol Chem. 2012.
Abstract
Hepatic insulin resistance has been attributed to both increased endoplasmic reticulum (ER) stress and accumulation of intracellular lipids, specifically diacylglycerol (DAG). The ER stress response protein, X-box-binding protein-1 (XBP1), was recently shown to regulate hepatic lipogenesis, suggesting that hepatic insulin resistance in models of ER stress may result from defective lipid storage, as opposed to ER-specific stress signals. Studies were designed to dissociate liver lipid accumulation and activation of ER stress signaling pathways, which would allow us to delineate the individual contributions of ER stress and hepatic lipid content to the pathogenesis of hepatic insulin resistance. Conditional XBP1 knock-out (XBP1Δ) and control mice were fed fructose chow for 1 week. Determinants of whole-body energy balance, weight, and composition were determined. Hepatic lipids including triglyceride, DAGs, and ceramide were measured, alongside markers of ER stress. Whole-body and tissue-specific insulin sensitivity were determined by hyperinsulinemic-euglycemic clamp studies. Hepatic ER stress signaling was increased in fructose chow-fed XBP1Δ mice as reflected by increased phosphorylated eIF2α, HSPA5 mRNA, and a 2-fold increase in hepatic JNK activity. Despite JNK activation, XBP1Δ displayed increased hepatic insulin sensitivity during hyperinsulinemic-euglycemic clamp studies, which was associated with increased insulin-stimulated IRS2 tyrosine phosphorylation, reduced hepatic DAG content, and reduced PKCε activity. These studies demonstrate that ER stress and IRE1α-mediated JNK activation can be disassociated from hepatic insulin resistance and support the hypothesis that hepatic insulin resistance in models of ER stress may be secondary to ER stress modulation of hepatic lipogenesis.
Figures
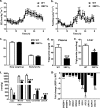
FIGURE 1.
XBP1Δ mice fed fructose chow have reduced hepatic lipid levels and expression of lipogenic genes. a, 24-h energy expenditure (EE) during fructose chow feeding. b, 24-h feeding during fructose feeding. c, body weight and fat mass after 1 week of fructose feeding. d, plasma triglyceride levels. e, hepatic triglyceride levels. f, hepatic DAG levels in cytosol (Cyto) and membrane (Memb) compartments, and ceramide and LCCoA levels. g, quantitative PCR of cDNA from liver of genes involved in lipid metabolism. The data are expressed as fold change relative to WT. (n = 6–8 for both genotypes aged 14–16 weeks for each panel). *, p < 0.05; **, p < 0.001; ***, p < 0.001.
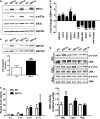
FIGURE 2.
Markers of ER stress are elevated in fructose chow-fed XBP1Δ mice. a, Western blots of liver lysates for ER stress markers IRE1α, phosphorylated and total eIF2α, and GAPDH loading control. b, quantitative PCR of cDNA isolated from liver for genes involved in the ER stress response. The data are reported as fold change relative to WT. c, Western blot of liver lysate for the ER chaperone GRP78 and GAPDH loading control. d, hepatic JNK activity from whole cell liver lysates (WC), cytoplasmic (Cyto), and nuclear (Nuc) fractions obtained by differential centrifugation. e, plasma levels of the cytokines IL1-β, IL-6, IL-10, and IL-12. TNFα and INFγ were below the level of detection (2.5 pg/ml) (n = 6–8 for both genotypes for each panel). The blots shown are representative of six to eight mice per genotype. *, p < 0.05; **, p < 0.001; ***, p < 0.001.
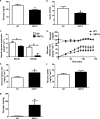
FIGURE 3.
Hepatic insulin sensitivity is improved in fructose chow-fed XBP1Δ mice. a, plasma glucose levels after a 14-h overnight fast. b, plasma insulin levels after a 14-h overnight fast. c, basal and insulin-stimulated (clamp) hepatic glucose production. d, plasma glucose levels (upper panel) and glucose infusion rate (lower panel) during hyperinsulinemic euglycemic clamps. e, glucose infusion rate required to maintain euglycemia during the final 40 min of the clamp. f, whole-body glucose uptake measured over the final 40 min of the clamp. g, hepatic glycogen levels measured following hyperinsulinemic euglycemic infusion (n = 6–9 for both genotypes for each panel). *, p < 0.05.
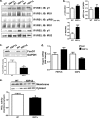
FIGURE 4.
Decreased PKCϵ activity is associated with improved insulin signaling in fructose chow-fed XBP1Δ mice. a, immunoprecipitation of liver lysates from clamped mice for the indicated antibodies, followed by Western blotting. IP, immunoprecipitation; IB, immunoblot. b, quantification of Western blotting data in A. c, total FoxO1 protein levels in insulin-treated (post-clamp) liver. d, hepatic G6PC and PEPCK mRNA levels in insulin-treated (post-clamp) liver determined by QPCR. e, PKCϵ activity in post-clamp liver determined as the ratio of membrane to cytosolic levels of PKCϵ. *, p < 0.05 (n = 6–9 for both genotypes for each panel). The blots shown are representative of six to nine mice per genotype. *, p < 0.05.
Similar articles
- Constitutive role for IRE1α-XBP1 signaling pathway in the insulin-mediated hepatic lipogenic program.
Ning J, Hong T, Ward A, Pi J, Liu Z, Liu HY, Cao W. Ning J, et al. Endocrinology. 2011 Jun;152(6):2247-55. doi: 10.1210/en.2010-1036. Epub 2011 Mar 29. Endocrinology. 2011. PMID: 21447637 Free PMC article. - Involvement of IRE1α signaling in the hippocampus in patients with mesial temporal lobe epilepsy.
Liu G, Guo H, Guo C, Zhao S, Gong D, Zhao Y. Liu G, et al. Brain Res Bull. 2011 Jan 15;84(1):94-102. doi: 10.1016/j.brainresbull.2010.10.004. Epub 2010 Oct 20. Brain Res Bull. 2011. PMID: 20965234 - Protein tyrosine phosphatase 1B and insulin resistance: role of endoplasmic reticulum stress/reactive oxygen species/nuclear factor kappa B axis.
Panzhinskiy E, Ren J, Nair S. Panzhinskiy E, et al. PLoS One. 2013 Oct 18;8(10):e77228. doi: 10.1371/journal.pone.0077228. eCollection 2013. PLoS One. 2013. PMID: 24204775 Free PMC article. Retracted. - Stressed out about obesity: IRE1α-XBP1 in metabolic disorders.
Sha H, He Y, Yang L, Qi L. Sha H, et al. Trends Endocrinol Metab. 2011 Sep;22(9):374-81. doi: 10.1016/j.tem.2011.05.002. Epub 2011 Jun 22. Trends Endocrinol Metab. 2011. PMID: 21703863 Free PMC article. Review. - Involvement of the IRE1α-XBP1 pathway and XBP1s-dependent transcriptional reprogramming in metabolic diseases.
Wu R, Zhang QH, Lu YJ, Ren K, Yi GH. Wu R, et al. DNA Cell Biol. 2015 Jan;34(1):6-18. doi: 10.1089/dna.2014.2552. DNA Cell Biol. 2015. PMID: 25216212 Free PMC article. Review.
Cited by
- Ankyrin-B metabolic syndrome combines age-dependent adiposity with pancreatic β cell insufficiency.
Lorenzo DN, Healy JA, Hostettler J, Davis J, Yang J, Wang C, Hohmeier HE, Zhang M, Bennett V. Lorenzo DN, et al. J Clin Invest. 2015 Aug 3;125(8):3087-102. doi: 10.1172/JCI81317. Epub 2015 Jul 13. J Clin Invest. 2015. PMID: 26168218 Free PMC article. - Dissociation of Muscle Insulin Resistance from Alterations in Mitochondrial Substrate Preference.
Song JD, Alves TC, Befroy DE, Perry RJ, Mason GF, Zhang XM, Munk A, Zhang Y, Zhang D, Cline GW, Rothman DL, Petersen KF, Shulman GI. Song JD, et al. Cell Metab. 2020 Nov 3;32(5):726-735.e5. doi: 10.1016/j.cmet.2020.09.008. Epub 2020 Oct 8. Cell Metab. 2020. PMID: 33035493 Free PMC article. - Endoplasmic reticulum proteostasis in hepatic steatosis.
Baiceanu A, Mesdom P, Lagouge M, Foufelle F. Baiceanu A, et al. Nat Rev Endocrinol. 2016 Dec;12(12):710-722. doi: 10.1038/nrendo.2016.124. Epub 2016 Aug 12. Nat Rev Endocrinol. 2016. PMID: 27516341 Review. - Emerging Pharmacological Targets for the Treatment of Nonalcoholic Fatty Liver Disease, Insulin Resistance, and Type 2 Diabetes.
Goedeke L, Perry RJ, Shulman GI. Goedeke L, et al. Annu Rev Pharmacol Toxicol. 2019 Jan 6;59:65-87. doi: 10.1146/annurev-pharmtox-010716-104727. Annu Rev Pharmacol Toxicol. 2019. PMID: 30625285 Free PMC article. Review. - Disruption of calcium transfer from ER to mitochondria links alterations of mitochondria-associated ER membrane integrity to hepatic insulin resistance.
Rieusset J, Fauconnier J, Paillard M, Belaidi E, Tubbs E, Chauvin MA, Durand A, Bravard A, Teixeira G, Bartosch B, Michelet M, Theurey P, Vial G, Demion M, Blond E, Zoulim F, Gomez L, Vidal H, Lacampagne A, Ovize M. Rieusset J, et al. Diabetologia. 2016 Mar;59(3):614-23. doi: 10.1007/s00125-015-3829-8. Epub 2015 Dec 10. Diabetologia. 2016. PMID: 26660890
References
- Holland W. L., Miller R. A., Wang Z. V., Sun K., Barth B. M., Bui H. H., Davis K. E., Bikman B. T., Halberg N., Rutkowski J. M., Wade M. R., Tenorio V. M., Kuo M. S., Brozinick J. T., Zhang B. B., Birnbaum M. J., Summers S. A., Scherer P. E. (2011) Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 - PMC - PubMed
- Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Görgün C., Glimcher L. H., Hotamisligil G. S. (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-082448/DK/NIDDK NIH HHS/United States
- R01 DK-40936/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- P30 DK-45735/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- U24 DK-059635/DK/NIDDK NIH HHS/United States
- R01 DK082448/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous