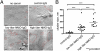Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes - PubMed (original) (raw)
Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes
Shannon R Hinson et al. Proc Natl Acad Sci U S A. 2012.
Abstract
The astrocytic aquaporin-4 (AQP4) water channel is the target of pathogenic antibodies in a spectrum of relapsing autoimmune inflammatory central nervous system disorders of varying severity that is unified by detection of the serum biomarker neuromyelitis optica (NMO)-IgG. Neuromyelitis optica is the most severe of these disorders. The two major AQP4 isoforms, M1 and M23, have identical extracellular residues. This report identifies two novel properties of NMO-IgG as determinants of pathogenicity. First, the binding of NMO-IgG to the ectodomain of astrocytic AQP4 has isoform-specific outcomes. M1 is completely internalized, but M23 resists internalization and is aggregated into larger-order orthogonal arrays of particles that activate complement more effectively than M1 when bound by NMO-IgG. Second, NMO-IgG binding to either isoform impairs water flux directly, independently of antigen down-regulation. We identified, in nondestructive central nervous system lesions of two NMO patients, two previously unappreciated histopathological correlates supporting the clinical relevance of our in vitro findings: (i) reactive astrocytes with persistent foci of surface AQP4 and (ii) vacuolation in adjacent myelin consistent with edema. The multiple molecular outcomes identified as a consequence of NMO-IgG interaction with AQP4 plausibly account for the diverse pathological features of NMO: edema, inflammation, demyelination, and necrosis. Differences in the nature and anatomical distribution of NMO lesions, and in the clinical and imaging manifestations of disease documented in pediatric and adult patients, may be influenced by regional and maturational differences in the ratio of M1 to M23 proteins in astrocytic membranes.
Conflict of interest statement
Conflict of interest statement: V.A.L. is a named inventor on a patent relating to AQP4 as a target of pathogenic autoantibodies in NMO and related disorders and on a pending patent related to AQP4 applications to cancer; has received greater than the federal threshold for significant interest from licensing of this technology; and receives no royalties from the sale of Mayo Medical Laboratories’ service serological tests. However, Mayo Collaborative Services, Inc., receives revenue for conducting these tests. In addition, V.A.L. and S.R.H are named inventors on two patent applications filed by the Mayo Foundation for Medical Education and Research relating to functional assays for detecting NMO/AQP4 antibody.
Figures
Fig. 1.
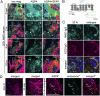
Internalization of AQP4 is incomplete in astrocytes exposed to NMO-IgG. (A) AQP4 (cyan) and GFAP intermediate filament (magenta) visualized by immunofluorescence before serum exposure (time 0) and after exposure to NMO patient serum. Boxed areas in low-magnification column are enlarged in the adjacent columns. (B) AQP4 domains recognized by IgG probes: Rabbit ECD-C-IgG binds to extracellular loop C, and mouse and rabbit AQP4-IgGs bind to intracellular C-terminal residues. N-terminal residues 1–22 are unique to M1. (C) AQP4 distribution in fetal rat astrocytes exposed live to NMO patient serum after 20 h. NMO-IgG (cyan) colocalizes with rabbit ECD-C IgG (magenta). Boxed areas designate regions enlarged in right column. (D) Localization of AQP4 (magenta) and the early endosomal antigen EEA1 (cyan) before and 10 min after exposure to NMO patient serum. Colocalized probes appear yellow (arrows). Asterisks indicate enlarged boxed areas from merged image. DNA is blue in all merged images. Displayed images represent data from three independent experiments (A and C) and from two independent experiments (D). (Scale bars, 20 μm, pertain to all panels of equal magnification.)
Fig. 2.
NMO-IgG binding internalizes M1 more efficiently than M23. (A) Distribution of AQP4 in HEK293 cells exogenously expressing M1 or M23 before and after exposure (37 °C) to NMO patient serum and representative of three experiments. Boxed region is enlarged at right. (Scale bars, 20 μm.) (B) Western blot shows AQP4 in lysates of HEK293 cells expressing M1 or M23. Actin serves as protein loading control. (C) Western blot shows AQP4 in lysates of astrocytes and control HEK293 cells. Astrocytes predominantly express M23. Exposure to NMO serum reduces M1 more than M23 (asterisk: extended autoradiographic exposure). (D) Quantification of three Western blot experiments. Bars indicate relative amounts of M1 and M23 remaining after exposure to NMO serum relative to control serum (*P = 0.003).
Fig. 3.
(A) Freeze-fracture electron microscopic analysis of mouse astrocytes cultured without and with IgG from control or NMO serum. Unexposed or exposed to control human IgG (∼2 mg/mL), most intramembranous particles are single and distributed uniformly. OAPs (encircled in red) are small and relatively sparse. After exposure for 24 h to IgG prepared from “low-titer” NMO serum (AQP4-binding capacity = 20 nmol/L), OAPs are modestly larger. After exposure to “high-titer” serum IgG (AQP4-binding capacity = 105 nmol/L), OAPs are much larger. (Scale bars, 100 nm.) (B) Scatter plot analysis of OAP size after exposure to control-IgG or NMO-IgG of low or high titer. Horizontal bars correspond to mean values. Differences between values for control-IgG and low-titer NMO-IgG and control-IgG and high-titer NMO-IgG and between low-titer and high-titer NMO-IgG are significant (***P = 0.0001). Differences for OAP cluster sizes in control-IgG and low-titer and high-titer NMO-IgG were compared by the Mann–Whitney test with GraphPad software.
Fig. 4.
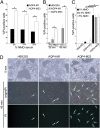
M23 interaction with NMO-IgG activates complement more effectively than M1. Simultaneous addition of complement and heat-inactivated NMO serum caused rapid lysis of HEK293 cells expressing M1 or M23. Therefore, for flow cytometric analysis cells were exposed sequentially to serum (30 min at 22 °C) and complement (45 min at 37 °C) (4). Lesioning (PI permeability) was negligible with heat-inactivated complement (values subtracted). Bars represent SEMs of three or more experiments. (A) Cells exposed to graded concentrations of NMO serum and 20% complement. Membrane lesioning was maximal with 5% NMO serum [M1 compared with M23; *P < 0.001 (5%); *P < 0.001 (10%); *P = 0.013 (20%)]. (B) To circumvent M1 internalization by NMO-IgG at 37 °C, we simultaneously added 5% serum and complement at suboptimal concentration (15%). M23 cells showed consistently greater vulnerability to lesioning [*P = 0.031 (30 min); *P = 0.013 (45 min)]. (C) With complement limited to 10% concentration for 45 min, its activation by M23 cells was consistently fourfold more than by M1 cells at all tested NMO serum concentrations [*P = 0.05 (1%); *P = 0.001 (2.5%); *P = 0.005 (5%)]. (D) Phase-contrast microscopy appearance of PI permeable cells (cyan; arrows) under conditions shown in B (nontransfected and transfected with M1 or M23). (Scale bar, 200 μm.)
Fig. 5.
NMO-IgG binding inhibits water influx through AQP4. (A) Western blot analysis shows relative levels of AQP4 protein in cRNA-injected oocytes. Each lane represents lysates from approximately four oocytes. (B) Immunofluorescence staining of M1, M23, and AQP1 in plasma membranes of cRNA-injected oocytes exposed to control-IgG or NMO-IgG. At 4 °C, regardless of human IgG, and at 22 °C with control human IgG, all AQP immunoreactivities are tightly confined to the plasma membrane. At 22 °C with NMO-IgG, AQP1 is unchanged, but AQP4 M1 and M23 immunoreactivities are more punctate (asterisks), and M1 is visibly associated with subplasmalemmal vesicles (arrows). (Scale bar, 10 μm, pertains to all panels.) (C) Representative hypotonic stress assay (immersion in distilled water) shows oocyte lysis times (seconds) after exposure to control-IgG or NMO-IgG for 4 h at 22 °C. Each condition included 5–12 injected oocytes. Injected cRNAs are indicated below the graph. Asterisks indicate significance in comparing oocytes exposed to control-IgG and NMO-IgG for M1 (P < 0.001), M23 (P = 0.005), and M1 + M23 (P < 0.001). (D and E) Cumulative data from five to six hypotonic stress assays show fold increase in lysis times for oocytes exposed to NMO-IgG relative to lysis times for oocytes exposed to control-IgG at 22 °C or 4 °C. (D) Asterisks indicate P values relative to AQP1 controls: M1 (P < 0.001), M23 (P < 0.001), M1 + M23 (P < 0.001), and relative to M23—M1 (P = 0.032) and M1 + M23 (P = 0.016). (E) Comparisons are relative to AQP1: M1 (P = 0.028) and M23 (P = 0.001); differences between M1 and M23 were not significant.
Fig. 6.
Immunohistopathological observations in NMO lesions reveal nonlytic astrocytic changes compatible with newly documented in vitro outcomes of NMO-IgG interacting with AQP4. Brain tissues from two seropositive NMO patients reveal incomplete internalization of surface AQP4 in astrocytes and tissue vacuolation. Forebrain autopsy (A_–_D) and cerebellar biopsy (E and F) with immunoperoxidase/hematoxylin stain. (A) NMO lesion (patient 1) shows characteristic loss of AQP4 immunoreactivity (brown) (Scale bar, 500 μm.) (B) Reactive astrocytes with incomplete AQP4 internalization (arrows) adjacent to blood vessels in the same NMO lesion (Scale bar, 33 μm.) (C) Some astrocytes in this lesion (arrows) also have AQP4 in cytoplasmic vesicles. Reactive astrocytes with complete AQP4 loss (arrowhead) are also visible (Scale bar, 50 μm.) (D) Higher magnification of C shows lesional astrocytes with partial AQP4 internalization (black arrows), or complete AQP4 loss (arrowhead), and an astrocyte with abundant surface AQP4 at a lesion edge (white arrow). (Scale bar, 33 μm.) (E) Myelin vacuolation in cerebellum of patient 2 (proteolipid protein immunoreactivity) (Scale bar, 50 μm.) (F) Same lesion as in E shows AQP4 partial internalization in an astrocyte (arrow) (Scale bar, 20 μm.)
Comment in
- Illuminating neuromyelitis optica pathogenesis.
Ransohoff RM. Ransohoff RM. Proc Natl Acad Sci U S A. 2012 Jan 24;109(4):1001-2. doi: 10.1073/pnas.1119288109. Epub 2012 Jan 23. Proc Natl Acad Sci U S A. 2012. PMID: 22308524 Free PMC article. No abstract available. - Consequences of NMO-IgG binding to aquaporin-4 in neuromyelitis optica.
Rossi A, Ratelade J, Papadopoulos MC, Bennett JL, Verkman AS. Rossi A, et al. Proc Natl Acad Sci U S A. 2012 Jun 12;109(24):E1511; author reply E1512. doi: 10.1073/pnas.1203463109. Epub 2012 May 15. Proc Natl Acad Sci U S A. 2012. PMID: 22589299 Free PMC article. No abstract available.
Similar articles
- Neuromyelitis optica IgG does not alter aquaporin-4 water permeability, plasma membrane M1/M23 isoform content, or supramolecular assembly.
Rossi A, Ratelade J, Papadopoulos MC, Bennett JL, Verkman AS. Rossi A, et al. Glia. 2012 Dec;60(12):2027-39. doi: 10.1002/glia.22417. Epub 2012 Sep 14. Glia. 2012. PMID: 22987455 Free PMC article. - Neuromyelitis Optica Immunoglobulin G present in sera from neuromyelitis optica patients affects aquaporin-4 expression and water permeability of the astrocyte plasma membrane.
Melamud L, Fernández JM, Rivarola V, Di Giusto G, Ford P, Villa A, Capurro C. Melamud L, et al. J Neurosci Res. 2012 Jun;90(6):1240-8. doi: 10.1002/jnr.22822. Epub 2012 Feb 22. J Neurosci Res. 2012. PMID: 22354518 - Astrocytic autoantibody of neuromyelitis optica (NMO-IgG) binds to aquaporin-4 extracellular loops, monomers, tetramers and high order arrays.
Iorio R, Fryer JP, Hinson SR, Fallier-Becker P, Wolburg H, Pittock SJ, Lennon VA. Iorio R, et al. J Autoimmun. 2013 Feb;40:21-7. doi: 10.1016/j.jaut.2012.07.008. Epub 2012 Aug 18. J Autoimmun. 2013. PMID: 22906356 Free PMC article. - The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica.
Lucchinetti CF, Guo Y, Popescu BF, Fujihara K, Itoyama Y, Misu T. Lucchinetti CF, et al. Brain Pathol. 2014 Jan;24(1):83-97. doi: 10.1111/bpa.12099. Brain Pathol. 2014. PMID: 24345222 Free PMC article. Review. - Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO.
Verkman AS, Phuan PW, Asavapanumas N, Tradtrantip L. Verkman AS, et al. Brain Pathol. 2013 Nov;23(6):684-95. doi: 10.1111/bpa.12085. Brain Pathol. 2013. PMID: 24118484 Free PMC article. Review.
Cited by
- Brain-reactive antibodies and disease.
Diamond B, Honig G, Mader S, Brimberg L, Volpe BT. Diamond B, et al. Annu Rev Immunol. 2013;31:345-85. doi: 10.1146/annurev-immunol-020711-075041. Annu Rev Immunol. 2013. PMID: 23516983 Free PMC article. Review. - The immune pathogenesis of multiple sclerosis.
Weissert R. Weissert R. J Neuroimmune Pharmacol. 2013 Sep;8(4):857-66. doi: 10.1007/s11481-013-9467-3. Epub 2013 May 10. J Neuroimmune Pharmacol. 2013. PMID: 23660832 Review. - Association of Pain with Plasma C5a in Patients with Neuromyelitis Optica Spectrum Disorders During Remission.
Tong Y, Liu J, Yang T, Wang J, Zhao T, Kang Y, Fan Y. Tong Y, et al. Neuropsychiatr Dis Treat. 2022 May 17;18:1039-1046. doi: 10.2147/NDT.S359620. eCollection 2022. Neuropsychiatr Dis Treat. 2022. PMID: 35615424 Free PMC article. - Spectrum of sublytic astrocytopathy in neuromyelitis optica.
Guo Y, Lennon VA, Parisi JE, Popescu B, Vasquez C, Pittock SJ, Howe CL, Lucchinetti CF. Guo Y, et al. Brain. 2022 May 24;145(4):1379-1390. doi: 10.1093/brain/awab394. Brain. 2022. PMID: 34718426 Free PMC article. - Experimental Models of Neuroimmunological Disorders: A Review.
da Silva APB, Silva RBM, Goi LDS, Molina RD, Machado DC, Sato DK. da Silva APB, et al. Front Neurol. 2020 May 12;11:389. doi: 10.3389/fneur.2020.00389. eCollection 2020. Front Neurol. 2020. PMID: 32477252 Free PMC article. Review.
References
- Hinson SR, McKeon A, Lennon VA. Neurological autoimmunity targeting aquaporin-4. Neuroscience. 2010;168:1009–1018. - PubMed
- Hinson SR, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–2231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases