Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non-demented elderly adults - PubMed (original) (raw)
Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non-demented elderly adults
Deepti Putcha et al. J Neurosci. 2011.
Abstract
Alzheimer's disease (AD) is associated with functional and structural alterations in a distributed network of brain regions supporting memory and other cognitive domains. Functional abnormalities are present in mild cognitive impairment (MCI) with evidence of early hyperactivity in medial temporal lobe regions, followed by failure of hippocampal activation as dementia develops. Atrophy in a consistent set of cortical regions, the "cortical signature of AD," has been reported at the stage of dementia, MCI, and even in clinically normal (CN) older individuals predicted to develop AD. Despite multiple lines of evidence for each of these findings, the relationship between this structural marker of AD-related neurodegeneration and this functional marker of the integrity of the episodic memory system has not yet been elucidated. We investigated this relationship in 34 nondemented older humans (CN, N = 18; MCI, N = 16). Consistent with previous studies, we found evidence of hippocampal hyperactivation in MCI compared with CN. Additionally, within this MCI group, increased hippocampal activation correlated with cortical thinning in AD-signature regions. Even within the CN group, increased hippocampal activity was negatively correlated with cortical thinning in a subset of regions, including the superior parietal lobule (r = -0.66; p < 0.01). These findings, across a continuum of nondemented and mildly impaired older adults, support the hypothesis that paradoxically increased hippocampal activity may be an early indicator of AD-related neurodegeneration in a distributed network.
Conflict of interest statement
Conflict of Interest: Dr. Sperling has consulted for Avid (unpaid), Bayer, Bristol-Myers-Squibb, Eisai, Elan, Janssen, and Pfizer, but not in a manner directly relevant to this manuscript.
Figures
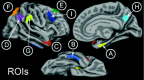
Figure 1.
AD signature of cortical thinning. A, Medial temporal lobe; B, inferior temporal gyrus; C, temporal pole; D, angular gyrus; E, superior frontal gyrus; F, superior parietal lobule; G, supramarginal gyrus; H, precuneus; I, inferior frontal sulcus.
Figure 2.
In MCI, memory test performance (percentage high confidence hits) is (a) negatively related to hippocampal activation (r = −0.43, p = 0.09) and (b) positively related to AD signature cortical thickness (r = 0.38, p = 0.03). Additionally, (c), Hippocampal activation is negatively related to hippocampal volume (r = −0.53, p = 0.04).
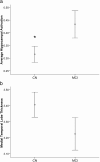
Figure 3.
CN (CDR 0) versus MCI (CDR 0.5) describing (a) hippocampal activation percentage signal change (t = 2.05, p = 0.04) and (b) medial temporal lobe cortical thickness in millimeters (b). * indicates a significant difference between the means.
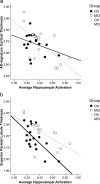
Figure 4.
Relationships by clinical subgroup between average hippocampal activation (percentage signal change) and cortical thickness in the (a) AD signature region summary measure (in millimeters) and (b) superior parietal lobule cortical thickness (in millimeters).
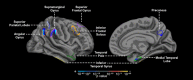
Figure 5.
ROI-restricted vertex-based thickness correlation map of hippocampal activation and cortical thickness (p < 0.01, uncorrected) with an AD signature mask applied in the whole sample.
Similar articles
- Fractionating the Rey Auditory Verbal Learning Test: Distinct roles of large-scale cortical networks in prodromal Alzheimer's disease.
Putcha D, Brickhouse M, Wolk DA, Dickerson BC; Alzheimer's Disease Neuroimaging Initiative. Putcha D, et al. Neuropsychologia. 2019 Jun;129:83-92. doi: 10.1016/j.neuropsychologia.2019.03.015. Epub 2019 Mar 28. Neuropsychologia. 2019. PMID: 30930301 Free PMC article. - Spatially distinct atrophy is linked to β-amyloid and tau in preclinical Alzheimer disease.
Wang L, Benzinger TL, Hassenstab J, Blazey T, Owen C, Liu J, Fagan AM, Morris JC, Ances BM. Wang L, et al. Neurology. 2015 Mar 24;84(12):1254-60. doi: 10.1212/WNL.0000000000001401. Epub 2015 Feb 25. Neurology. 2015. PMID: 25716355 Free PMC article. - Antisaccade task reflects cortical involvement in mild cognitive impairment.
Heuer HW, Mirsky JB, Kong EL, Dickerson BC, Miller BL, Kramer JH, Boxer AL. Heuer HW, et al. Neurology. 2013 Oct 1;81(14):1235-43. doi: 10.1212/WNL.0b013e3182a6cbfe. Epub 2013 Aug 28. Neurology. 2013. PMID: 23986300 Free PMC article. - Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer's disease in people with mild cognitive impairment.
Lombardi G, Crescioli G, Cavedo E, Lucenteforte E, Casazza G, Bellatorre AG, Lista C, Costantino G, Frisoni G, Virgili G, Filippini G. Lombardi G, et al. Cochrane Database Syst Rev. 2020 Mar 2;3(3):CD009628. doi: 10.1002/14651858.CD009628.pub2. Cochrane Database Syst Rev. 2020. PMID: 32119112 Free PMC article. - Disease tracking markers for Alzheimer's disease at the prodromal (MCI) stage.
Drago V, Babiloni C, Bartrés-Faz D, Caroli A, Bosch B, Hensch T, Didic M, Klafki HW, Pievani M, Jovicich J, Venturi L, Spitzer P, Vecchio F, Schoenknecht P, Wiltfang J, Redolfi A, Forloni G, Blin O, Irving E, Davis C, Hårdemark HG, Frisoni GB. Drago V, et al. J Alzheimers Dis. 2011;26 Suppl 3:159-99. doi: 10.3233/JAD-2011-0043. J Alzheimers Dis. 2011. PMID: 21971460 Review.
Cited by
- Disrupted Brain Structural Connectivity: Pathological Interactions Between Genetic APOE ε4 Status and Developed MCI Condition.
Ma C, Wang J, Zhang J, Chen K, Li X, Shu N, Chen Y, Liu Z, Zhang Z. Ma C, et al. Mol Neurobiol. 2017 Nov;54(9):6999-7007. doi: 10.1007/s12035-016-0224-5. Epub 2016 Oct 26. Mol Neurobiol. 2017. PMID: 27785756 - Pattern separation and pattern completion in Alzheimer's disease: evidence of rapid forgetting in amnestic mild cognitive impairment.
Ally BA, Hussey EP, Ko PC, Molitor RJ. Ally BA, et al. Hippocampus. 2013 Dec;23(12):1246-58. doi: 10.1002/hipo.22162. Epub 2013 Aug 14. Hippocampus. 2013. PMID: 23804525 Free PMC article. - Episodic Memory, Hippocampal Volume, and Function for Classification of Mild Cognitive Impairment Patients Regarding Amyloid Pathology.
Miotto EC, Brucki SMD, Cerqueira CT, Bazán PR, Silva GAA, Martin MDGM, da Silveira PS, Faria DP, Coutinho AM, Buchpiguel CA, Busatto Filho G, Nitrini R. Miotto EC, et al. J Alzheimers Dis. 2022;89(1):181-192. doi: 10.3233/JAD-220100. J Alzheimers Dis. 2022. PMID: 35871330 Free PMC article. - Brodmann area 10: Collating, integrating and high level processing of nociception and pain.
Peng K, Steele SC, Becerra L, Borsook D. Peng K, et al. Prog Neurobiol. 2018 Feb;161:1-22. doi: 10.1016/j.pneurobio.2017.11.004. Epub 2017 Dec 2. Prog Neurobiol. 2018. PMID: 29199137 Free PMC article. Review. - Modulation of Brain Hyperexcitability: Potential New Therapeutic Approaches in Alzheimer's Disease.
Toniolo S, Sen A, Husain M. Toniolo S, et al. Int J Mol Sci. 2020 Dec 7;21(23):9318. doi: 10.3390/ijms21239318. Int J Mol Sci. 2020. PMID: 33297460 Free PMC article. Review.
References
- Bobinski M, de Leon MJ, Convit A, De Santi S, Wegiel J, Tarshish CY, Saint Louis LA, Wisniewski HM. MRI of entorhinal cortex in mild Alzheimer's disease. Lancet. 1999;353:38–40. - PubMed
Publication types
MeSH terms
Grants and funding
- K24 AG035007/AG/NIA NIH HHS/United States
- R01 AG-027435/AG/NIA NIH HHS/United States
- R01 AG027435/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- K23 AG027171/AG/NIA NIH HHS/United States
- K23 AG033634/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical