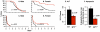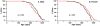Does reduced IGF-1R signaling in Igf1r+/- mice alter aging? - PubMed (original) (raw)
doi: 10.1371/journal.pone.0026891. Epub 2011 Nov 23.
Neha Garg, Yuji Ikeno, Sachin Thakur, Nicolas Musi, Ralph A DeFronzo, Ning Zhang, Rebecca C Erickson, Jon Gelfond, Gene B Hubbard, Martin L Adamo, Arlan Richardson
Affiliations
- PMID: 22132081
- PMCID: PMC3223158
- DOI: 10.1371/journal.pone.0026891
Does reduced IGF-1R signaling in Igf1r+/- mice alter aging?
Alex F Bokov et al. PLoS One. 2011.
Abstract
Mutations in insulin/IGF-1 signaling pathway have been shown to lead to increased longevity in various invertebrate models. Therefore, the effect of the haplo-insufficiency of the IGF-1 receptor (Igf1r(+/-)) on longevity/aging was evaluated in C57Bl/6 mice using rigorous criteria where lifespan and end-of-life pathology were measured under optimal husbandry conditions using large sample sizes. Igf1r(+/-) mice exhibited reductions in IGF-1 receptor levels and the activation of Akt by IGF-1, with no compensatory increases in serum IGF-1 or tissue IGF-1 mRNA levels, indicating that the Igf1r(+/-) mice show reduced IGF-1 signaling. Aged male, but not female Igf1r(+/-) mice were glucose intolerant, and both genders developed insulin resistance as they aged. Female, but not male Igf1r(+/-) mice survived longer than wild type mice after lethal paraquat and diquat exposure, and female Igf1r(+/-) mice also exhibited less diquat-induced liver damage. However, no significant difference between the lifespans of the male Igf1r(+/-) and wild type mice was observed; and the mean lifespan of the Igf1r(+/-) females was increased only slightly (less than 5%) compared to wild type mice. A comprehensive pathological analysis showed no significant difference in end-of-life pathological lesions between the Igf1r(+/-) and wild type mice. These data show that the Igf1r(+/-) mouse is not a model of increased longevity and delayed aging as predicted by invertebrate models with mutations in the insulin/IGF-1 signaling pathway.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
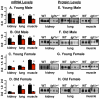
Figure 1. Igf1rβ expression.
The mRNA and protein levels of the β subunit of Igf1r were measured in the kidney, lung, and muscle (quadriceps) from male and female mice that were 6 (Graphs A, E, C, and G) and 25 (Graphs B, F, D, and H) months old. The graphs on the left represent data from qRT-PCR and those on the right represent data from Western blots. Three to 6 animals were used per group. Black bars represent WT mice and red bars represent Igf1r+/− mice; the mean and SEM are shown, and asterisks indicate tissues showing a difference between WT and Igf1r+/− where p<0.05. The Student's t-test was used for the comparisons.
Figure 2. Induction of AKT phosphorylation by IGF-1 in WT and Igf-1r+/− mice.
Levels of phosphorylated AKT were measured in the muscle (quadriceps) of 6- (graphs A and C) and 25- (graphs B and D) month-old male and female mice following injection of saline or rhIGF-1 (1 mg/kg body wt.) using Western blots as described in Materials and Methods. Three to 4 animals were used per group.
Figure 3. Induction of GSK3β phosphorylation and levels of Igfbp5 mRNA transcript in WT and Igf1r+/− mice.
Levels of phosphorylated GSK3β were measured in the muscle (quadriceps) of 25-month-old male (graph A) and female (graph B) mice following injection of saline or rhIGF-1 (1 mg/kg body wt.) using Western blots as described in Materials and Methods. Three animals were used per group. The expression of Igfbp5 was measured in the same samples using qRT-PCR (graphs C and D). The vertical axis represents expression levels relative to B2M and the error bars represent SEM. P-values of 0.01 and 0.005 are represented by * and **, respectively.
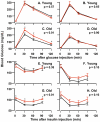
Figure 4. Glucose and Insulin Tolerance Tests.
GTTs (2 g/kg, i.p.) were performed in 6-month-old male (A) and female (B) as well as 25-month-old male (C) and female (D) mice after a 12 hr fast, and blood glucose was recorded at times indicated. ITTs (0.5 U/kg, i.p.) were performed in 6-month-old male (E) and female (F) as well as 25-month-old male (G) and female (H) mice after a 5 hr fast. The data were obtained from 6 to 8 animals per group and the SEM is shown. Black lines show the blood glucose levels of WT (black lines) and Igf1r+/− mice (red lines). The Student's t-test was used for the comparisons of the areas under the curve (AUC) and the p-values are shown on each graph. Individual points were also compared in the same manner and corrected for multiple comparisons, and corrected p-values less than 0.05 are denoted by asterisks.
Figure 5. Peripheral Insulin Sensitivity.
Peripheral (muscle) insulin sensitivity was measured with a 90 min hyperinsulinemic euglycemic clamp performed in 4 WT and 5 Igf1r+/− females, all 25 months old. Bars represent the average ± SE glucose infusion rate during the last 20 min of the clamp. A student's t-test was used to compare the infusion rates between the two groups, averaged over the 20 min period for each animal.
Figure 6. Sensitivity of WT and Igf1r+/− mice to oxidative stress.
Paraquat (50 mg/kg) was administered to 37 male WT mice and 26 male Igf1r+/− mice (graph A) as well as to 39 female WT mice and 24 female _Igf1r+/−_mice (graph B). Diquat (50 mg/kg) was administered to 21 WT and 26 Igf1r+/− male mice (graph C) as well as to 22 WT and 17 Igf1r+/− female mice (graph D). The mice in graphs A–D were 5 to 9.5 months of age. Censored data points (due to uncertainty about the exact minute of an animal's death) are indicated by vertical tick-marks. Graphs E and F: Female WT and Igf1r+/− mice (10 to 11 months of age) were treated with diquat (50 mg/kg). Six hours after treatment the mice were killed and the ALT activities in the plasma (graph E) and number of apoptotic cells per unit area in a liver cross section (graph F) of 7 WT and 8 _Igf1r+/−_mice were determined. The mean and SEM are shown in the bar graphs. Black represents WT data and red represents _Igf1r+/−_data. Survival data were analyzed using the log-rank test while the ALT and apoptosis data were analyzed using Student's t-test and the p-values are shown.
Figure 7. Longevity of WT and Igf1r+/− mice.
The survival curves of 55 WT and 52 Igf1r+/− male mice (A) and 68 WT and 47 Igf1r+/− female (B) mice are shown in black for WT mice and red for _Igf1r+/−_mice. The survival curves were compared using the log-rank test, and the p-values are shown.
Similar articles
- Impaired IGF1R signaling in cells expressing longevity-associated human IGF1R alleles.
Tazearslan C, Huang J, Barzilai N, Suh Y. Tazearslan C, et al. Aging Cell. 2011 Jun;10(3):551-4. doi: 10.1111/j.1474-9726.2011.00697.x. Epub 2011 Apr 12. Aging Cell. 2011. PMID: 21388493 Free PMC article. - A viral insulin-like peptide inhibits IGF-1 receptor phosphorylation and regulates IGF1R gene expression.
Chrudinová M, Kirk NS, Chuard A, Venugopal H, Zhang F, Lubos M, Gelfanov V, Páníková T, Žáková L, Cutone J, Mojares M, DiMarchi R, Jiráček J, Altindis E. Chrudinová M, et al. Mol Metab. 2024 Feb;80:101863. doi: 10.1016/j.molmet.2023.101863. Epub 2024 Jan 3. Mol Metab. 2024. PMID: 38182007 Free PMC article. - Insulin-like growth factor binding protein-4 and -5 modulate ligand-dependent estrogen receptor-α activation in breast cancer cells in an IGF-independent manner.
Hermani A, Shukla A, Medunjanin S, Werner H, Mayer D. Hermani A, et al. Cell Signal. 2013 Jun;25(6):1395-402. doi: 10.1016/j.cellsig.2013.02.018. Epub 2013 Mar 14. Cell Signal. 2013. PMID: 23499909 - [Insulin/IGF-1 signaling and aging].
Sasako T, Ueki K. Sasako T, et al. Nihon Rinsho. 2016 Sep;74(9):1435-1440. Nihon Rinsho. 2016. PMID: 30557473 Review. Japanese. - Insulin/IGF-1 and ROS signaling pathway cross-talk in aging and longevity determination.
Papaconstantinou J. Papaconstantinou J. Mol Cell Endocrinol. 2009 Feb 5;299(1):89-100. doi: 10.1016/j.mce.2008.11.025. Epub 2008 Dec 3. Mol Cell Endocrinol. 2009. PMID: 19103250 Free PMC article. Review.
Cited by
- Genetic differences and longevity-related phenotypes influence lifespan and lifespan variation in a sex-specific manner in mice.
Yuan R, Musters CJM, Zhu Y, Evans TR, Sun Y, Chesler EJ, Peters LL, Harrison DE, Bartke A. Yuan R, et al. Aging Cell. 2020 Nov;19(11):e13263. doi: 10.1111/acel.13263. Epub 2020 Oct 26. Aging Cell. 2020. PMID: 33105070 Free PMC article. - Growth signaling and longevity in mouse models.
Kim SS, Lee CK. Kim SS, et al. BMB Rep. 2019 Jan;52(1):70-85. doi: 10.5483/BMBRep.2019.52.1.299. BMB Rep. 2019. PMID: 30545442 Free PMC article. Review. - Exercise and the Cisd2 Prolongevity Gene: Two Promising Strategies to Delay the Aging of Skeletal Muscle.
Teng YC, Wang JY, Chi YH, Tsai TF. Teng YC, et al. Int J Mol Sci. 2020 Nov 28;21(23):9059. doi: 10.3390/ijms21239059. Int J Mol Sci. 2020. PMID: 33260577 Free PMC article. Review. - Evaluating Health Span in Preclinical Models of Aging and Disease: Guidelines, Challenges, and Opportunities for Geroscience.
Huffman DM, Justice JN, Stout MB, Kirkland JL, Barzilai N, Austad SN. Huffman DM, et al. J Gerontol A Biol Sci Med Sci. 2016 Nov;71(11):1395-1406. doi: 10.1093/gerona/glw106. Epub 2016 Aug 16. J Gerontol A Biol Sci Med Sci. 2016. PMID: 27535967 Free PMC article. - Sex-specific alterations in glucose homeostasis and metabolic parameters during ageing of caspase-2-deficient mice.
Wilson CH, Nikolic A, Kentish SJ, Shalini S, Hatzinikolas G, Page AJ, Dorstyn L, Kumar S. Wilson CH, et al. Cell Death Discov. 2016 Feb 29;2:16009. doi: 10.1038/cddiscovery.2016.9. eCollection 2016. Cell Death Discov. 2016. PMID: 27551503 Free PMC article.
References
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. - PubMed
- Klass M. A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mechanisms of Ageing and Development. 1983;22:279–286. - PubMed
- Riddle DL. The Nematode Caenorhabditis elegans. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory; 1988. The Dauer Larva. pp. 393–414.
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. - PubMed
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans 7. Science. 1997;277:942–946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AG021890/AG/NIA NIH HHS/United States
- R01 DK024092/DK/NIDDK NIH HHS/United States
- P01 AG019316/AG/NIA NIH HHS/United States
- P30 AG013319/AG/NIA NIH HHS/United States
- DK-80157/DK/NIDDK NIH HHS/United States
- R01AG026012/AG/NIA NIH HHS/United States
- AG-26557/AG/NIA NIH HHS/United States
- DK-24092/DK/NIDDK NIH HHS/United States
- DK-089229/DK/NIDDK NIH HHS/United States
- AG-13319/AG/NIA NIH HHS/United States
- 1P30-AG-13319/AG/NIA NIH HHS/United States
- R37 AG026557/AG/NIA NIH HHS/United States
- R01 AG023843/AG/NIA NIH HHS/United States
- K23 AG030979/AG/NIA NIH HHS/United States
- R01 AG026012/AG/NIA NIH HHS/United States
- AG-23843/AG/NIA NIH HHS/United States
- AG-19316/AG/NIA NIH HHS/United States
- R01 DK089229/DK/NIDDK NIH HHS/United States
- AG-030979/AG/NIA NIH HHS/United States
- R01 DK080157/DK/NIDDK NIH HHS/United States
- AGO-21890/PHS HHS/United States
- R56 DK024092/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous