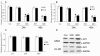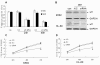miR-15a and miR-16-1 inhibit the proliferation of leukemic cells by down-regulating WT1 protein level - PubMed (original) (raw)
miR-15a and miR-16-1 inhibit the proliferation of leukemic cells by down-regulating WT1 protein level
Shen-meng Gao et al. J Exp Clin Cancer Res. 2011.
Abstract
Background: miR-15a and miR-16-1(miR-15a/16-1) have been implicated as tumor suppressors in chronic lymphocytic leukemia, multiple myeloma, and acute myeloid leukemic cells. However the mechanism of inhibiting the proliferation of leukemic cells is poorly understood.
Methods: K562 and HL-60 cells were transfected with pRS-15/16 or pRS-E, cell growth were measured by CCK-8 assay and direct cell count. Meanwhile WT1 protein and mRNA level were measured by Western blotting and quantitative real-time PCR.
Results: In this study we found that over-expression of miR-15a/16-1 significantly inhibited K562 and HL-60 cells proliferation. Enforced expression of miR-15a/16-1 in K562 and HL-60 cells significantly reduced the protein level of WT1 but not affected the mRNA level. However enforced expression of miR-15a/16-1 can not reduce the activity of a luciferase reporter carrying the 3'-untranslated region(3'UTR) of WT1. Silencing of WT1 by specific siRNA suppressed leukemic cells proliferation resembling that of miR-15a/16-1 over-expression. Anti-miR-15a/16-1 oligonucleotides (AMO) reversed the expression of WT1 in K562 and HL-60 cells. Finally, we found a significant inverse correlation between miR-15a or miR-16-1 expression and WT1 protein levels in primary acute myeloid leukemia (AML) blasts and normal controls.
Conclusions: These data suggest that miR-15a/16-1 may function as a tumor suppressor to regulate leukemic cell proliferation potentially by down-regulating the WT1 oncogene. However WT1 is not directly targeted by miR-15a/16-1 through miRNA-mRNA base pairing, therefore more study are required to understand the mechanism by which miR-15a/16-1 downregulate WT1.
Figures
Figure 1
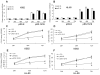
Effects of miR-15a/16-1 on the proliferation of K562 and HL-60 cells. K562 and HL-60 cells were transfected with pRS-15/16 or pRS-E vector (negative control) for 24, 48 and 72 hours, then the relative expressions of miR-15a/16-1 were measured by qRT-PCR (A and B). CCK-8 assay (C and E) and direct cell count (D and F) were performed when K562 and HL-60 cells were transfected with pRS-15/16 or pRS-E vector at different time periods. Data are shown as mean ± SD from three independent experiments. *P < 0.05 versus negative control.
Figure 2
miR-15a/16-1 downregulates WT1 protein level not through targeting mRNAs according to the degree of complementarity with their 3'UTR. (A) K562 and HL-60 cells were transiently transfected with pRS-15/16 or pRS-E vector for different time periods and subjected to western analysis with the indicated antibodies. The level of GAPDH was used as a loading control. (B) K562 and HL-60 cells were transfected with pRS-15/16 or pRS-E vector for 24 and 48 hours, then the relative expression of WT1 was measured by quantitative real-time PCR. (C and D). K562 and HL-60 cells were transfected with the pGL-3 containing Bcl-2 3'UTR or WT1 3'UTR and pRS-15/16 or pRS-E for 24 hours, relative repression fold of firefly luciferase expression was standardized to Renilla luciferase, pGL-TK.
Figure 3
AMO-miR-15a/16-1 reversed the expression of WT1 in K562 and HL-60 cells (A) and (B) AMO inhibited the expression of miR-15a/16-1. K562 and HL-60 cells were incubated with AMO-miR-15a/16-1 for 24 and 48 hours, then miR-15a/16-1 and U6 snRNA expression were determined by quantitative real-time PCR. (C) and (D) K562 and HL-60 cells were incubated with AMO-miR-15a/16-1 for 48 h, then mRNA and protein level of WT1 were detected by quantitative real-time PCR and Western blotting individually. *P < 0.01 versus SCR.
Figure 4
The role of miR-15a/16-1 in the regulation of leukemic cell proliferation. (A) and (B) K562 and HL-60 cells were incubated with 1.5 ug siRNA-WT1, 1.5 ug N.C or neither of the above for 24 and 48 hours, then the relative expression of WT1 and the corresponding WT1 protein level were separately measured by quantitative real-time PCR and Western blotting. (C) and (D) K562 and HL-60 cells treated with siRNA or N.C or neither of the above were measured by CCK-8 assay at different time periods. Data are shown as mean ± SD from three independent experiments. *P < 0.05 versus negative control.
Figure 5
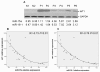
WT1 protein expression is inversely correlated with miR-15a or miR-16-1 expression in AML samples and normal controls. (A) WT1 protein levels from 2 normal controls (N1 and N2) and 6 AML samples (P1-P6) were measured by Western blotting. The numbers represent the relative expression of miR-15a and miR-16-1 in the same specimens. (B) and (C) Inverse correlation between miR-15a or miR-16-1 expression and WT1 protein level in 25 primary AML samples and 5 normal controls. A statistically significant correlation between miR-15a or miR-16-1 expression and WT1 protein level was observed by Pearson's method. WT1 verse miR-15a R = -0.73 P < 0.01; WT1 verse miR-16-1 R = -0.76 P < 0.01
Similar articles
- Pure curcumin decreases the expression of WT1 by upregulation of miR-15a and miR-16-1 in leukemic cells.
Gao SM, Yang JJ, Chen CQ, Chen JJ, Ye LP, Wang LY, Wu JB, Xing CY, Yu K. Gao SM, et al. J Exp Clin Cancer Res. 2012 Mar 27;31(1):27. doi: 10.1186/1756-9966-31-27. J Exp Clin Cancer Res. 2012. PMID: 22449094 Free PMC article. - Curcumin attenuates Adriamycin-resistance of acute myeloid leukemia by inhibiting the lncRNA HOTAIR/miR-20a-5p/WT1 axis.
Liu JM, Li M, Luo W, Sun HB. Liu JM, et al. Lab Invest. 2021 Oct;101(10):1308-1317. doi: 10.1038/s41374-021-00640-3. Epub 2021 Jul 19. Lab Invest. 2021. PMID: 34282279 - A regulatory circuitry between miR-193a/miR-600 and WT1 enhances leukemogenesis in acute myeloid leukemia.
Li H, Xing C, Zhou B, Ye H, Feng J, Wu J, Gao S. Li H, et al. Exp Hematol. 2018 May;61:59-68.e5. doi: 10.1016/j.exphem.2018.02.001. Epub 2018 Feb 13. Exp Hematol. 2018. PMID: 29452230 - miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma.
Chava S, Reynolds CP, Pathania AS, Gorantla S, Poluektova LY, Coulter DW, Gupta SC, Pandey MK, Challagundla KB. Chava S, et al. Mol Oncol. 2020 Jan;14(1):180-196. doi: 10.1002/1878-0261.12588. Epub 2019 Nov 29. Mol Oncol. 2020. PMID: 31637848 Free PMC article. - miR-15a-5p suppresses endometrial cancer cell growth via Wnt/β-catenin signaling pathway by inhibiting WNT3A.
Wang ZM, Wan XH, Sang GY, Zhao JD, Zhu QY, Wang DM. Wang ZM, et al. Eur Rev Med Pharmacol Sci. 2017 Nov;21(21):4810-4818. Eur Rev Med Pharmacol Sci. 2017. PMID: 29164582
Cited by
- Combined transfection of Bcl-2 siRNA and miR-15a oligonucleotides enhanced methotrexate-induced apoptosis in Raji cells.
Ding L, Hu XM, Wu H, Liu GX, Gao YJ, He DM, Zhang Y. Ding L, et al. Cancer Biol Med. 2013 Mar;10(1):16-21. doi: 10.7497/j.issn.2095-3941.2013.01.003. Cancer Biol Med. 2013. PMID: 23691440 Free PMC article. - Concise Review: Chronic Myeloid Leukemia: Stem Cell Niche and Response to Pharmacologic Treatment.
Arrigoni E, Del Re M, Galimberti S, Restante G, Rofi E, Crucitta S, Baratè C, Petrini M, Danesi R, Di Paolo A. Arrigoni E, et al. Stem Cells Transl Med. 2018 Mar;7(3):305-314. doi: 10.1002/sctm.17-0175. Epub 2018 Feb 8. Stem Cells Transl Med. 2018. PMID: 29418079 Free PMC article. Review. - The CXCR4 inhibitor BL-8040 induces the apoptosis of AML blasts by downregulating ERK, BCL-2, MCL-1 and cyclin-D1 via altered miR-15a/16-1 expression.
Abraham M, Klein S, Bulvik B, Wald H, Weiss ID, Olam D, Weiss L, Beider K, Eizenberg O, Wald O, Galun E, Avigdor A, Benjamini O, Nagler A, Pereg Y, Tavor S, Peled A. Abraham M, et al. Leukemia. 2017 Nov;31(11):2336-2346. doi: 10.1038/leu.2017.82. Epub 2017 Mar 10. Leukemia. 2017. PMID: 28280274 - Deficiency in WT1-targeting microRNA-125a leads to myeloid malignancies and urogenital abnormalities.
Tatsumi N, Hojo N, Yamada O, Ogawa M, Katsura Y, Kawata S, Morii E, Sakamoto H, Inaba R, Tsuda A, Fukuda I, Moriguchi N, Hasuwa H, Okabe M, Fujiki F, Nishida S, Nakajima H, Tsuboi A, Oka Y, Hosen N, Sugiyama H, Oji Y. Tatsumi N, et al. Oncogene. 2016 Feb 25;35(8):1003-14. doi: 10.1038/onc.2015.154. Epub 2015 May 11. Oncogene. 2016. PMID: 25961914 - Downregulation of miR-486-5p contributes to tumor progression and metastasis by targeting protumorigenic ARHGAP5 in lung cancer.
Wang J, Tian X, Han R, Zhang X, Wang X, Shen H, Xue L, Liu Y, Yan X, Shen J, Mannoor K, Deepak J, Donahue JM, Stass SA, Xing L, Jiang F. Wang J, et al. Oncogene. 2014 Feb 27;33(9):1181-9. doi: 10.1038/onc.2013.42. Epub 2013 Mar 11. Oncogene. 2014. PMID: 23474761 Free PMC article.
References
- Garzon R, Pichiorri F, Palumbo T, Visentini M, Aqeilan R, Cimmino A, Wang H, Sun H, Volinia S, Alder H, Calin GA, Liu CG, Andreeff M, Croce CM. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148–4157. doi: 10.1038/sj.onc.1210186. - DOI - PubMed
- Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical