Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells - PubMed (original) (raw)
Review
Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells
Ben Youngblood et al. Curr Opin HIV AIDS. 2012 Jan.
Abstract
Purpose of review: Failure to control viral infections such as HIV results in T-cell receptor (TCR) and inhibitory receptor driven exhaustion of antigen-specific T cells. Persistent signaling by these receptors during chronic viral infection sculpts the transcriptional regulatory programs of virus-specific T cells. The resulting gene expression profile is tailored to temper the potentially damaging effector functions of cytotoxic T cells and adapt them to an antigen-rich and inflammation-rich environment. Here we review recent studies investigating mechanisms of transcriptional regulation of effector, functional memory, and exhausted T-cell functions during acute versus chronic infections.
Recent findings: Patterns of gene expression in virus-specific CD8 T cells are a result of a combination of pro and inhibitory signals from antigen presentation (TCR-mediated) and co-inhibitory receptor ligation (PD-1, 2B4). Further, memory-specific transcriptional regulation of 2B4 expression and signaling impose a self-limiting secondary effector response to a prolonged viral infection. Additionally, differentiation of functional memory CD8 T cells is coupled with acquisition of a repressive epigenetic program for PD-1 expression. However, chronic infection provides a signal that blocks the acquisition of these epigenetic modifications reinforcing the suppression of cytotoxic lymphocyte (CTL) functions in exhausted cells.
Summary: Current findings suggest that the mechanism(s) that delineate functional memory versus exhaustion are coupled with acquisition of transcriptional programs at the effector stage of differentiation, reinforced by cessation or persistence of TCR signaling.
Figures
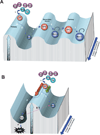
Figure 1
Rolling hill diagram of antigen-mediated transcriptional reprogramming of CD8 T cells during memory differentiation. A) Differentiation of antigen-specific CD8 T cells during primary, secondary, and tertiary acute viral infection. B) Differentiation of antigen-specific CD8 T cells during chronic viral infection versus acute viral infection. The peaks and troughs of the transcriptional landscape of the Y-axis represent the potential to achieve an effector response through the modification of transcriptional programs. The Z-axis (blue arrow) represents the duration of antigen exposure and the lineage commitment of the antigen-specific CD8 T cell. Step 1: Antigen-specific naïve CD8 T cells encounter an antigen-presenting cell and differentiate into cytolytic effector cells. Step 2: During the ~ 105 fold expansion of effector cells a subset of cells commit to a memory fate. Step 3: Following antigen clearance the effector population contracts, with ~5–10% of the population surviving to differentiate into resting memory CD8 T cells, poised to rapidly recall the effector functions. Step 4: Memory CD8 T cells efficiently recall an effector transcriptional profile upon secondary exposure to the antigen or a vaccine boost regimen. Step 5: Secondary effector CD8 T cells undergo less contraction resulting in a greater quantity of self-renewing memory CD8 T cells with a gene expression profile that retains some effector-like transcriptional status. Steps 6 & 7: The resulting memory population generated from a tertiary infection are more effector-like and increased in quantity relative to the primary and secondary memory cells. Step 3’: If the infection persists the antigen-specific CD8 T cells further differentiate with progressive restriction in the ability to recall effector functions and ultimately loss of the virus-specific cells. Step 8: Future efforts will determine if exhausted CD8 T cells can be reprogrammed to obtain the transcriptional regulation of a resting memory CD8 T cell.
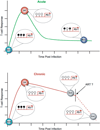
Figure 2
Epigenetic modifications at the PD-1 locus are coupled to persistence of antigen. During an acute viral infection (green line) the PD-1 locus in early effector antigen-specific CD8 T cells becomes unmethylated (open lollipops) and the chromatin more accessible relative to naïve and memory CD8 T cells. Functional memory CD8 T cells have reacquire a unique DNA methylation pattern (filled lollipops) relative to their naïve precursors. Exhausted CD8 T cells retain an unmethylated and accessible PD-1 locus during chronic viral infection (red line). Anti-retroviral therapies can reduce viral load, but it remains to be determined if the PD-1 locus becomes remethylated in exhausted virus-specific CD8 T cells.
Similar articles
- Transcription Factor IRF4 Promotes CD8+ T Cell Exhaustion and Limits the Development of Memory-like T Cells during Chronic Infection.
Man K, Gabriel SS, Liao Y, Gloury R, Preston S, Henstridge DC, Pellegrini M, Zehn D, Berberich-Siebelt F, Febbraio MA, Shi W, Kallies A. Man K, et al. Immunity. 2017 Dec 19;47(6):1129-1141.e5. doi: 10.1016/j.immuni.2017.11.021. Epub 2017 Dec 12. Immunity. 2017. PMID: 29246443 - T Cell Receptor Diversity and Lineage Relationship between Virus-Specific CD8 T Cell Subsets during Chronic Lymphocytic Choriomeningitis Virus Infection.
Chang YM, Wieland A, Li ZR, Im SJ, McGuire DJ, Kissick HT, Antia R, Ahmed R. Chang YM, et al. J Virol. 2020 Sep 29;94(20):e00935-20. doi: 10.1128/JVI.00935-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32759317 Free PMC article. - PD-L1 Checkpoint Inhibition Narrows the Antigen-Specific T Cell Receptor Repertoire in Chronic Lymphocytic Choriomeningitis Virus Infection.
Klein S, Ghersi D, Manns MP, Prinz I, Cornberg M, Kraft ARM. Klein S, et al. J Virol. 2020 Aug 31;94(18):e00795-20. doi: 10.1128/JVI.00795-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641478 Free PMC article. - Transcriptional regulatory network for the establishment of CD8+ T cell exhaustion.
Seo W, Jerin C, Nishikawa H. Seo W, et al. Exp Mol Med. 2021 Feb;53(2):202-209. doi: 10.1038/s12276-021-00568-0. Epub 2021 Feb 24. Exp Mol Med. 2021. PMID: 33627794 Free PMC article. Review. - CD8+ T cell exhaustion.
Kurachi M. Kurachi M. Semin Immunopathol. 2019 May;41(3):327-337. doi: 10.1007/s00281-019-00744-5. Epub 2019 Apr 15. Semin Immunopathol. 2019. PMID: 30989321 Review.
Cited by
- T-Cell Receptor (TCR) Clonotype-Specific Differences in Inhibitory Activity of HIV-1 Cytotoxic T-Cell Clones Is Not Mediated by TCR Alone.
Flerin NC, Chen H, Glover TD, Lamothe PA, Zheng JH, Fang JW, Ndhlovu ZM, Newell EW, Davis MM, Walker BD, Goldstein H. Flerin NC, et al. J Virol. 2017 Feb 28;91(6):e02412-16. doi: 10.1128/JVI.02412-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077649 Free PMC article. - Antigen-Presenting Intratumoral B Cells Affect CD4+ TIL Phenotypes in Non-Small Cell Lung Cancer Patients.
Bruno TC, Ebner PJ, Moore BL, Squalls OG, Waugh KA, Eruslanov EB, Singhal S, Mitchell JD, Franklin WA, Merrick DT, McCarter MD, Palmer BE, Kern JA, Slansky JE. Bruno TC, et al. Cancer Immunol Res. 2017 Oct;5(10):898-907. doi: 10.1158/2326-6066.CIR-17-0075. Epub 2017 Aug 28. Cancer Immunol Res. 2017. PMID: 28848053 Free PMC article. - Activation-induced cell death drives profound lung CD4(+) T-cell depletion in HIV-associated chronic obstructive pulmonary disease.
Popescu I, Drummond MB, Gama L, Coon T, Merlo CA, Wise RA, Clements JE, Kirk GD, McDyer JF. Popescu I, et al. Am J Respir Crit Care Med. 2014 Oct 1;190(7):744-55. doi: 10.1164/rccm.201407-1226OC. Am J Respir Crit Care Med. 2014. PMID: 25137293 Free PMC article. - Selective Loss of Signaling Lymphocytic Activation Molecule Family Member 4-Positive CD8+ T Cells Contributes to the Decreased Cytotoxic Cell Activity in Systemic Lupus Erythematosus.
Kis-Toth K, Comte D, Karampetsou MP, Kyttaris VC, Kannan L, Terhorst C, Tsokos GC. Kis-Toth K, et al. Arthritis Rheumatol. 2016 Jan;68(1):164-73. doi: 10.1002/art.39410. Arthritis Rheumatol. 2016. PMID: 26314831 Free PMC article. - Immunologic Aging in Adults with Congenital Heart Disease: Does Infant Sternotomy Matter?
Elder RW, George RP, McCabe NM, Rodriguez FH III, Book WM, Mahle WT, Kirk AD. Elder RW, et al. Pediatr Cardiol. 2015 Oct;36(7):1411-6. doi: 10.1007/s00246-015-1174-9. Epub 2015 Apr 28. Pediatr Cardiol. 2015. PMID: 25916315 Free PMC article.
References
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science (New York, N.Y. 1996;272:54–60. - PubMed
- Bevan MJ, Goldrath AW. T-cell memory: You must remember this. Curr Biol. 2000;10:R338–R340. - PubMed
- Doherty PC, Topham DJ, Tripp RA. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunological reviews. 1996;150:23–44. - PubMed
- Lefrancois L, Masopust D. T cell immunity in lymphoid and non-lymphoid tissues. Current opinion in immunology. 2002;14:503–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI083022/AI/NIAID NIH HHS/United States
- AI083022/AI/NIAID NIH HHS/United States
- AI078897/AI/NIAID NIH HHS/United States
- P01 AI080192/AI/NIAID NIH HHS/United States
- AI095608/AI/NIAID NIH HHS/United States
- U01 AI095608/AI/NIAID NIH HHS/United States
- R01 AI030048/AI/NIAID NIH HHS/United States
- AI082630/AI/NIAID NIH HHS/United States
- R01 AI071309/AI/NIAID NIH HHS/United States
- U19 AI082630/AI/NIAID NIH HHS/United States
- P01 AI078897/AI/NIAID NIH HHS/United States
- AI071309/AI/NIAID NIH HHS/United States
- HHSN266200500030C/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials