The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats - PubMed (original) (raw)
The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats
Evelyn Jantscher-Krenn et al. Gut. 2012 Oct.
Abstract
Background: Necrotising enterocolitis (NEC) is one of the most common and fatal intestinal disorders in preterm infants. Breast-fed infants are at lower risk for NEC than formula-fed infants, but the protective components in human milk have not been identified. In contrast to formula, human milk contains high amounts of complex glycans.
Objective: To test the hypothesis that human milk oligosaccharides (HMO) contribute to the protection from NEC.
Methods: Since human intervention studies are unfeasible due to limited availability of HMO, a neonatal rat NEC model was used. Pups were orally gavaged with formula without and with HMO and exposed to hypoxia episodes. Ileum sections were scored blindly for signs of NEC. Two-dimensional chromatography was used to determine the most effective HMO, and sequential exoglycosidase digestions and linkage analysis was used to determine its structure.
Results: Compared to formula alone, pooled HMO significantly improved 96-hour survival from 73.1% to 95.0% and reduced pathology scores from 1.98 ± 1.11 to 0.44 ± 0.30 (p<0.001). Within the pooled HMO, a specific isomer of disialyllacto-N-tetraose (DSLNT) was identified to be protective. Galacto-oligosaccharides, currently added to formula to mimic some of the effects of HMO, had no effect.
Conclusion: HMO reduce NEC in neonatal rats and the effects are highly structure specific. If these results translate to NEC in humans, DSLNT could be used to prevent or treat NEC in formula-fed infants, and its concentration in the mother's milk could serve as a biomarker to identify breast-fed infants at risk of developing this disorder.
Conflict of interest statement
Competing interests None.
Figures
Figure 1
Human milk oligosaccharides (HMO) and galacto-oligosaccharides (GOS) are structurally different. (A) Fluorescence high-performance liquid chromatography (HPLC-FL) chromatogram of 2AB-labelled HMO isolated from pooled human milk. Most common HMO are annotated and listed in panel B. *Disialyllacto-N-tetraose (DSLNT), which was later identified as the necrotising enterocolitis-protective HMO. (B) Schematic representation of the most common oligosaccharides found in the isolated pooled HMO. Numbers in brackets correspond to the annotated peaks in panel A. 2′FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3′SL, 3′-sialyllactose; LNT, lacto-N-tetraose; LNnT, lacto-N-neotetraose; LNFP1, lacto-N-fucopentaose 1; LNFP2, lacto-N-fucopentaose 2; LSTb, sialyllacto-N-tetraose b; LSTc, sialyllacto-N-tetraose c. Monosaccharide key: dark circle, glucose (Glc); light circle, galactose (Gal); square, N-acetyl-glucosamine (GlcNAc); triangle, fucose (Fuc); diamond, N-acetyl-neuraminic acid (NeuAc). (C) HPLC-FL chromatogram of Vivinal GOS. Peak clusters represent structural isomers of oligosaccharides with the same degree of polymerisation and depend on the number of galactose residues per GOS molecule. Comparison of the HMO and GOS chromatograms confirmed a clear difference in the structural composition.
Figure 2
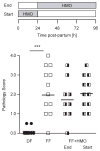
Pooled human milk oligosaccharides (HMO), but not galacto-oligosaccharides (GOS) improve survival and reduce necrotising enterocolitis (NEC) in neonatal rats. (A) Survival of neonatal rats within the first 96 h post-partum. DF, dam-fed; FF, formula-fed; FF+HMO, fed formula with HMO (10 mg/ml); FF+GOS, fed formula with GOS (8 mg/ml). (B) Macroscopic evaluation of rat intestines at 96 h post-partum. Compared to DF (left) and FF +HMO (right) animals, the intestines of FF animals (centre) were darker with patchy necrosis and evidence of haemorrhagic intestine as well as intramural gas cysts (Pneumatosis intestinalis). (C) Microscopic evaluation of H&E-stained rat ileum sections. Based on the presence or absence of histological anomalies (three examples are shown in the bottom panel), ileum sections were graded from 0 (normal) to 4 (complete destruction). (D) Ileum pathology scores at 96 h post-partum. Each intervention was tested in a total of 10–20 animals in three independent experiments. Each symbol represents the pathology score for an individual animal. Horizontal lines represent mean pathology scores. ***p<0.001.
Figure 3
Exposure to human milk oligosaccharides (HMO) in the first 24 h post-partum is required, but not sufficient to reduce necrotising enterocolitis. Neonatal rats were dam-fed (DF), fed HMO-free formula for the entire first 96 h post-partum (FF), fed HMO-free formula for the first 24 h and then switched to HMO-containing formula (10 mg/ml) for the remaining 72 h (FF+HMO End), or fed HMO-containing formula for the first 24 h and then switched to HMO-free formula (FF+HMO Start). Each intervention was tested in a total of 9–12 animals in two independent experiments. ***p<0.001.
Figure 4
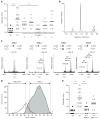
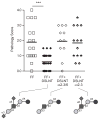
A single, disialylated human milk oligosaccharide (HMO) reduces necrotising enterocolitis. (A) Ileum pathology scores in response to adding charge-fractionated HMO to formula. Anion exchange chromatography was used to fractionate pooled HMO by charge based on whether HMO contained no (0), one (−1), two (−2), three (−3) or four (−4) sialic acid residues. The −2 charged HMO fraction, containing oligosaccharides with two sialic acids (two negative charges) had the most pronounced effect. (B) HPLC-FL chromatogram of −2 charged HMO fraction. (C) MALDI-TOF mass spectra and potential composition of the four major HMO peaks in the −2 charged HMO fraction. The predicted number of hexoses (circles), hexosamines (square), N-acetylneuramic acid (NeuAc, diamond) and fucose (triangle) per molecule are listed above each mass spectrum. Loss of NeuAc during analysis reduces the mass by 291 Da. (D) Fast protein liquid chromatography (FPLC) with a gel exclusion column was used to separate the four major HMO peaks in the −2 charged HMO fraction by size. FPLC fractions containing mostly HMO peak 2 were pooled together (HMO 2). HMO peaks 3 and 4 could not be separated by gel exclusion and were pooled in one fraction (HMO 3+4). (E) Ileum pathology scores in response to adding size-fractionated HMO to formula. Each intervention was tested in a total of 11–14 animals in two independent experiments. ***p<0.001.
Figure 5
The necrotising enterocolitis-protective human milk oligosaccharide (HMO) is disialyllacto-N-tetraose (DSLNT). (A) Linkage specific neuraminidase treatment shows the presence of one α2–3- and one α2–6-linked N-acetyl-neuraminic acid (NeuAc). Fluorescence high-performance liquid chromatography (HPLC-FL) chromatogram a: protective HMO 2; b: HMO 2 after treatment with α2–3-specific neuraminidase; c: HMO 2 after treatment with linkage promiscuous neuramidase. (B) The underlying HMO backbone has a type I structure (Galβ1–3GlcNAc). HPLC-FL chromatogram d: asialo-HMO 2 (after treatment with α2–3/6 neuraminidase, product c); e: asialo-HMO 2 after treatment with β1–3-specific galactosidase; f: asialo-HMO 2 after treatment with β1–4-specific galactosidase. (C) The subterminal sugar in the HMO backbone is N-acetyl-glucosamine (GlcNAc). HPLC-FL chromatogram g: asialo-agalacto-HMO 2 (after treatment with α2–3/6 neuraminidase and β1–3 galactosidase, product e); h: asialo-agalacto-HMO 2 after treatment with GlcNAcase. (D) Gas chromatography mass spectrum (GC–MS) of partially methylated alditol acetate (PMAA) derivatives of HMO 2. (E) Schematic representation of DSLNT based on the results from sequential exoglycosidase digestion and GC–MS PMAA linkage analysis.
Figure 6
Sialic acid is required for the necrotising enterocolitis (NEC) protective effects of disialyllacto-N-tetraose (DSLNT). Ileum pathology scores in response to adding DSLNT or neuraminidase-treated DSLNT to formula. Commercially available DSLNT (300 μM) significantly reduced NEC pathology scores. Treatment with a linkage promiscuous neuraminidase (α2–3/6) or an α2–3-specific neuraminidase abolished the protective effects of DSLNT. Each intervention was tested in a total of 11–26 animals in three independent experiments. ***p<0.001.
Similar articles
- Sialylated galacto-oligosaccharides and 2'-fucosyllactose reduce necrotising enterocolitis in neonatal rats.
Autran CA, Schoterman MH, Jantscher-Krenn E, Kamerling JP, Bode L. Autran CA, et al. Br J Nutr. 2016 Jul;116(2):294-9. doi: 10.1017/S0007114516002038. Epub 2016 May 23. Br J Nutr. 2016. PMID: 27212112 - Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants.
Autran CA, Kellman BP, Kim JH, Asztalos E, Blood AB, Spence ECH, Patel AL, Hou J, Lewis NE, Bode L. Autran CA, et al. Gut. 2018 Jun;67(6):1064-1070. doi: 10.1136/gutjnl-2016-312819. Epub 2017 Apr 5. Gut. 2018. PMID: 28381523 - Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis.
Masi AC, Embleton ND, Lamb CA, Young G, Granger CL, Najera J, Smith DP, Hoffman KL, Petrosino JF, Bode L, Berrington JE, Stewart CJ. Masi AC, et al. Gut. 2021 Dec;70(12):2273-2282. doi: 10.1136/gutjnl-2020-322771. Epub 2020 Dec 16. Gut. 2021. PMID: 33328245 Free PMC article. - Human Milk Oligosaccharides to Prevent Gut Dysfunction and Necrotizing Enterocolitis in Preterm Neonates.
Bering SB. Bering SB. Nutrients. 2018 Oct 8;10(10):1461. doi: 10.3390/nu10101461. Nutrients. 2018. PMID: 30297668 Free PMC article. Review. - Structure-function relationships of human milk oligosaccharides.
Bode L, Jantscher-Krenn E. Bode L, et al. Adv Nutr. 2012 May 1;3(3):383S-91S. doi: 10.3945/an.111.001404. Adv Nutr. 2012. PMID: 22585916 Free PMC article. Review.
Cited by
- Milk bioactives may manipulate microbes to mediate parent-offspring conflict.
Allen-Blevins CR, Sela DA, Hinde K. Allen-Blevins CR, et al. Evol Med Public Health. 2015 Apr 2;2015(1):106-21. doi: 10.1093/emph/eov007. Evol Med Public Health. 2015. PMID: 25835022 Free PMC article. Review. - A bacterial sialidase mediates early-life colonization by a pioneering gut commensal.
Buzun E, Hsu CY, Sejane K, Oles RE, Vasquez Ayala A, Loomis LR, Zhao J, Rossitto LA, McGrosso DM, Gonzalez DJ, Bode L, Chu H. Buzun E, et al. Cell Host Microbe. 2024 Feb 14;32(2):181-190.e9. doi: 10.1016/j.chom.2023.12.014. Epub 2024 Jan 15. Cell Host Microbe. 2024. PMID: 38228143 - Human Milk Oligosaccharides: Potential Applications in COVID-19.
Chutipongtanate S, Morrow AL, Newburg DS. Chutipongtanate S, et al. Biomedicines. 2022 Feb 1;10(2):346. doi: 10.3390/biomedicines10020346. Biomedicines. 2022. PMID: 35203555 Free PMC article. Review. - Human Milk Oligosaccharides Impact Cellular and Inflammatory Gene Expression and Immune Response.
Rosa F, Sharma AK, Gurung M, Casero D, Matazel K, Bode L, Simecka C, Elolimy AA, Tripp P, Randolph C, Hand TW, Williams KD, LeRoith T, Yeruva L. Rosa F, et al. Front Immunol. 2022 Jun 29;13:907529. doi: 10.3389/fimmu.2022.907529. eCollection 2022. Front Immunol. 2022. PMID: 35844612 Free PMC article. - Synthesis and Characterization of Sialylated Lactose- and Lactulose-Derived Oligosaccharides by Trypanosoma cruzi Trans-sialidase.
Pham HTT, Ten Kate GA, Dijkhuizen L, van Leeuwen SS. Pham HTT, et al. J Agric Food Chem. 2019 Mar 27;67(12):3469-3479. doi: 10.1021/acs.jafc.8b06974. Epub 2019 Mar 14. J Agric Food Chem. 2019. PMID: 30836749 Free PMC article.
References
- Uauy RD, Fanaroff AA, Korones SB, et al. Necrotising enterocolitis in very low birth weight infants: biodemographic and clinical correlates. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1991;119:630–8. - PubMed
- Holman RC, Stoll BJ, Curns AT, et al. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20:498–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R03 HD059717/HD/NICHD NIH HHS/United States
- K99 DK078668/DK/NIDDK NIH HHS/United States
- R03 HD059717S1/HD/NICHD NIH HHS/United States
- R21 AI083612/AI/NIAID NIH HHS/United States
- R01 AI014032/AI/NIAID NIH HHS/United States
- K99/R00 DK078668/DK/NIDDK NIH HHS/United States
- R00 DK078668/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources