Induction of antibodies in rhesus macaques that recognize a fusion-intermediate conformation of HIV-1 gp41 - PubMed (original) (raw)
doi: 10.1371/journal.pone.0027824. Epub 2011 Nov 30.
Laura L Sutherland, Frederick H Jaeger, Kara M Anasti, Robert Parks, Shelley Stewart, Cindy Bowman, Shi-Mao Xia, Ruijun Zhang, Xiaoying Shen, Richard M Scearce, Gilad Ofek, Yongping Yang, Peter D Kwong, Sampa Santra, Hua-Xin Liao, Georgia Tomaras, Norman L Letvin, Bing Chen, S Munir Alam, Barton F Haynes
Affiliations
- PMID: 22140469
- PMCID: PMC3227606
- DOI: 10.1371/journal.pone.0027824
Induction of antibodies in rhesus macaques that recognize a fusion-intermediate conformation of HIV-1 gp41
S Moses Dennison et al. PLoS One. 2011.
Abstract
A component to the problem of inducing broad neutralizing HIV-1 gp41 membrane proximal external region (MPER) antibodies is the need to focus the antibody response to the transiently exposed MPER pre-hairpin intermediate neutralization epitope. Here we describe a HIV-1 envelope (Env) gp140 oligomer prime followed by MPER peptide-liposomes boost strategy for eliciting serum antibody responses in rhesus macaques that bind to a gp41 fusion intermediate protein. This Env-liposome immunization strategy induced antibodies to the 2F5 neutralizing epitope ⁶⁶⁴DKW residues, and these antibodies preferentially bound to a gp41 fusion intermediate construct as well as to MPER scaffolds stabilized in the 2F5-bound conformation. However, no serum lipid binding activity was observed nor was serum neutralizing activity for HIV-1 pseudoviruses present. Nonetheless, the Env-liposome prime-boost immunization strategy induced antibodies that recognized a gp41 fusion intermediate protein and was successful in focusing the antibody response to the desired epitope.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
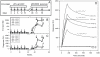
Figure 1. Comparison of gp41 MPER specific antibody responses induced in guinea pigs by JRFL gp140CF and MPER peptide-liposomes.
The immunization scheme of the study is shown in the top panel. A–B: The 2F5 epitope peptide (SP62 peptide) specific responses in guinea pigs sera (at 1∶50 dilution) determined by SPR are shown after vaccination with JRFL gp140CF (A) and MPER peptide-liposome (B). PB, pre-bleed; P1–P3, post-immune bleeds 1–3. C–D: SPR sensogram displaying the comparison of 2F5 epitope peptide specific binding of guinea pig 1402 (C) and 1703 (D) sera at different time points with 2F5 mAb is shown as representative data. The estimated dissociation rates (kd) from the sensogram are indicated for 2F5 mAb and Post-bleed 3 (P3). E: Epitope mapping of post-immune bleed 3 of guinea pigs 1402 (immunized with JRFL gp140CF alone) and 1702–1705 (immunized with MPER656 peptide-liposomes alone) is shown in comparison to 2F5 mAb. The normalized binding shown is the ratio between binding responses of sera to the alanine scanning mutant peptides and WT 2F5 epitope peptide SP62. Alanine substitution of residues that resulted in ≥50% reduction in binding are indicated adjacent to the symbols. Data is representative of at least two measurements on adjacent spots on the same sensor chip.
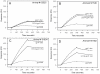
Figure 2. MPER specific immunogenicity and fine specificity of different prime-boost regimens in guinea pigs.
A–C: Immunization scheme (A), 2F5 epitope peptide (SP62 peptide) specific binding serum responses (B) and fine specificity of post immune bleed 7 (C) are shown for MPER656 liposome prime-JRFL gp140CF boost regimen are shown. PB, pre-bleed; P1–P7, post-immune bleeds 1–7. D–F: The JRFL gp140CF prime-MPER656 liposome boost regimen scheme (D), 2F5 epitope peptide specific binding serum responses € and the fine specificity of post immune bleed 4 (F) are shown. Data is representative of at least two measurements on adjacent spots on the same sensor chip.
Figure 3. Comparison of MPER specific immunogenicity of different immunogens in rhesus macaques.
The immunization scheme is shown at the top. A–B: MPER specific binding responses of sera from immunized rhesus macaques at different time points are shown for (A) JRFL gp140CF (ASO1B adjuvant) and (B) MPER peptide liposome immunogens (in emulsigen+oCpG). C–E: The fine specificities of MPER specific responses elicited by MPER liposomes in rhesus macaques are shown for post immunizations 2–5 sera. F: The alanine substituted peptides used in epitope mapping of MPER responses. The dotted ellipses in C, D and E highlight the predominance of 664D specific MPER responses. Data is representative of at least two measurements on adjacent spots on the same sensor chip.
Figure 4. MPER specific binding responses of Rhesus macaques sera in gp140 prime-MPER liposome boost regimen.
A: The immunization scheme involving JRFL gp140CF prime (ASO1B adjuvant) and MPER656/MPL-A/R848 (emulsigen+oCpG) liposome boost regimen is shown. The arrows indicate the weeks at which immunization was done. B: ELISA end point titer of the 2F5 epitope peptide specific responses in rhesus macaques sera at different time points. C: The 2F5 epitope peptide specific binding of monkeys sera determined by SPR is shown post vaccination at different time points. D: Representative data of SPR sensogram of rhesus macaque #41546 sera at different time points binding to 2F5 epitope peptide as compared to 2F5 mAb (10 µg/ml) is shown. The estimated dissociation rates (kd) are indicated for samples at week 51 and 56 as well as for the control 2F5 mAb. Data is representative of at least two measurements on adjacent spots on the same sensor chip.
Figure 5. Mapping of critical residues involved in MPER specific Rhesus macaques serum responses.
Normalized binding responses of immune sera from rhesus macaques primed with JRFL gp140CF and boosted with MPER656 liposomes. Panels A–D are for animals 03525, 41546, 51384 and 51902. The dotted circle highlights the mapping of MPER specific responses to D664KW residues as did 2F5 mAb. Data is representative of at least two measurements on adjacent spots on the same sensor chip.
Figure 6. The MPER specific serum responses in rhesus macaques recognize the mimic of fusion-intermediate gp41 structure.
A: Comparison of binding of 2F5 and 13H11 Fabs to GCN4-gp41-inter, a mimic of pre-hairpin intermediate structure of gp41. B–E: SPR sensogram of rhesus macaques sera (1∶50 diluted) binding to GCN4-gp41-inter. Each panel in B thru E compares the binding of sera obtained prior to MPER656 liposomes boost (week 48) and post-MPER656 liposomes boost (weeks 52–56). Data is representative of two measurements.
Figure 7. Comparison of JRFL gp140CF primed and MPER656 liposomes boosted rhesus macaque serum IgG binding to different conformational states of gp41.
A–D: SPR sensogram of rhesus macaques serum IgG (100 µg/ml) binding to GCN4-gp41 inter (solid lines), gp41-inter (dotted lines) and recombinant gp41 (broken lines). The IgGs purified from Week 56 (for animals 03525 and 51902), Week 52 (animal 41546) and Week 52 (animal 51834) sera were used. Data is representative of two measurements.
Figure 8. The JRFL gp140CF primed and MPER656 liposomes boosted rhesus macaque serum IgG bind scaffold proteins containing engrafted MPER in the 2F5 bound conformation.
A–C: SPR sensogram of 2F5, 11F10 and 13H11 mAbs at the indicated concentrations binding to ES2 (A), ES4 (B), and ES5 (C) scaffolds respectively are shown. D–F: The post MPER656 liposome immune serum IgG of rhesus macaques (150 µg/ml) binding to ES2 (D), E€(E), ES5 (F) are shown. For the sake of clarity, the scaffold binding responses of pre-MPER656 liposome serum IgG of only one animal (# 41546) is shown. The responses from other animal serum IgG were similar. Data is representative of at least two measurements.
Similar articles
- Envelope deglycosylation enhances antigenicity of HIV-1 gp41 epitopes for both broad neutralizing antibodies and their unmutated ancestor antibodies.
Ma BJ, Alam SM, Go EP, Lu X, Desaire H, Tomaras GD, Bowman C, Sutherland LL, Scearce RM, Santra S, Letvin NL, Kepler TB, Liao HX, Haynes BF. Ma BJ, et al. PLoS Pathog. 2011 Sep;7(9):e1002200. doi: 10.1371/journal.ppat.1002200. Epub 2011 Sep 1. PLoS Pathog. 2011. PMID: 21909262 Free PMC article. - A fusion intermediate gp41 immunogen elicits neutralizing antibodies to HIV-1.
Lai RP, Hock M, Radzimanowski J, Tonks P, Hulsik DL, Effantin G, Seilly DJ, Dreja H, Kliche A, Wagner R, Barnett SW, Tumba N, Morris L, LaBranche CC, Montefiori DC, Seaman MS, Heeney JL, Weissenhorn W. Lai RP, et al. J Biol Chem. 2014 Oct 24;289(43):29912-26. doi: 10.1074/jbc.M114.569566. Epub 2014 Aug 26. J Biol Chem. 2014. PMID: 25160627 Free PMC article. - Immunogens Modeling a Fusion-Intermediate Conformation of gp41 Elicit Antibodies to the Membrane Proximal External Region of the HIV Envelope Glycoprotein.
Vassell R, He Y, Vennakalanti P, Dey AK, Zhuang M, Wang W, Sun Y, Biron-Sorek Z, Srivastava IK, LaBranche CC, Montefiori DC, Barnett SW, Weiss CD. Vassell R, et al. PLoS One. 2015 Jun 18;10(6):e0128562. doi: 10.1371/journal.pone.0128562. eCollection 2015. PLoS One. 2015. PMID: 26087072 Free PMC article. - Neutralizing Antibodies Targeting HIV-1 gp41.
Caillat C, Guilligay D, Sulbaran G, Weissenhorn W. Caillat C, et al. Viruses. 2020 Oct 23;12(11):1210. doi: 10.3390/v12111210. Viruses. 2020. PMID: 33114242 Free PMC article. Review. - Antigp41 membrane proximal external region antibodies and the art of using the membrane for neutralization.
Cerutti N, Loredo-Varela JL, Caillat C, Weissenhorn W. Cerutti N, et al. Curr Opin HIV AIDS. 2017 May;12(3):250-256. doi: 10.1097/COH.0000000000000364. Curr Opin HIV AIDS. 2017. PMID: 28422789 Review.
Cited by
- Initiation of immune tolerance-controlled HIV gp41 neutralizing B cell lineages.
Zhang R, Verkoczy L, Wiehe K, Munir Alam S, Nicely NI, Santra S, Bradley T, Pemble CW 4th, Zhang J, Gao F, Montefiori DC, Bouton-Verville H, Kelsoe G, Larimore K, Greenberg PD, Parks R, Foulger A, Peel JN, Luo K, Lu X, Trama AM, Vandergrift N, Tomaras GD, Kepler TB, Moody MA, Liao HX, Haynes BF. Zhang R, et al. Sci Transl Med. 2016 Apr 27;8(336):336ra62. doi: 10.1126/scitranslmed.aaf0618. Sci Transl Med. 2016. PMID: 27122615 Free PMC article. - A Comprehensive Analysis of the T and B Lymphocytes Repertoire Shaped by HIV Vaccines.
Wang L, Zhang W, Lin L, Li X, Saksena NK, Wu J, Wang S, Joyce JG, Zhang X, Yang H, Wang J, Wang IM, Liu X. Wang L, et al. Front Immunol. 2018 Sep 26;9:2194. doi: 10.3389/fimmu.2018.02194. eCollection 2018. Front Immunol. 2018. PMID: 30319643 Free PMC article. - Structure and immunogenicity of a peptide vaccine, including the complete HIV-1 gp41 2F5 epitope: implications for antibody recognition mechanism and immunogen design.
Serrano S, Araujo A, Apellániz B, Bryson S, Carravilla P, de la Arada I, Huarte N, Rujas E, Pai EF, Arrondo JLR, Domene C, Jiménez MA, Nieva JL. Serrano S, et al. J Biol Chem. 2014 Mar 7;289(10):6565-6580. doi: 10.1074/jbc.M113.527747. Epub 2014 Jan 15. J Biol Chem. 2014. PMID: 24429284 Free PMC article. - HIV-1 antibodies and vaccine antigen selectively interact with lipid domains.
Hardy GJ, Wong GC, Nayak R, Anasti K, Hirtz M, Shapter JG, Alam SM, Zauscher S. Hardy GJ, et al. Biochim Biophys Acta. 2014 Oct;1838(10):2662-9. doi: 10.1016/j.bbamem.2014.07.007. Epub 2014 Jul 11. Biochim Biophys Acta. 2014. PMID: 25019685 Free PMC article. - How HIV-1 entry mechanism and broadly neutralizing antibodies guide structure-based vaccine design.
Pancera M, Changela A, Kwong PD. Pancera M, et al. Curr Opin HIV AIDS. 2017 May;12(3):229-240. doi: 10.1097/COH.0000000000000360. Curr Opin HIV AIDS. 2017. PMID: 28422787 Free PMC article. Review.
References
- Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, et al. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. - PubMed
- Julien JP, Bryson S, Nieva JL, Pai EF. Structural details of HIV-1 recognition by the broadly neutralizing monoclonal antibody 2F5: epitope conformation, antigen-recognition loop mobility, and anion-binding site. J Mol Biol. 2008;384:377–392. - PubMed
- Stiegler G, Kunert R, Purtscher M, Wolbank S, Voglauer R, et al. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2001;17:1757–1765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources