Heterogeneity of astrocytic form and function - PubMed (original) (raw)
Review
Heterogeneity of astrocytic form and function
Nancy Ann Oberheim et al. Methods Mol Biol. 2012.
Abstract
Astrocytes participate in all essential CNS functions, including blood flow regulation, energy metabolism, ion and water homeostasis, immune defence, neurotransmission, and adult neurogenesis. It is thus not surprising that astrocytic morphology and function differ between regions, and that different subclasses of astrocytes exist within the same brain region. Recent lines of work also show that the complexity of protoplasmic astrocytes increases during evolution. Human astrocytes are structurally more complex, larger, and propagate calcium signals significantly faster than rodent astrocytes. In this chapter, we review the diversity of astrocytic form and function, while considering the markedly expanded roles of astrocytes with phylogenetic evolution. We also define major challenges for the future, which include determining how astrocytic functions are locally specified, defining the molecular controls upon astrocytic fate and physiology and establishing how evolutionary changes in astrocytes contribute to higher cognitive functions.
Figures
Fig. 1
Prototypical astrocytic morphologies. (a) Cajal's drawing of astrocytes (indicated by “A”) in the pyramidal layer of the human hippocampus (indicated by “D”), twin astrocytes (indicated by “B”) and a satellite cell called the “third element” by Cajal (indicated by “a”). Sublimated gold chloride method. (b) Different astrocytes (indicated by “A,” “B,” “C” and “D”) surrounding neuronal somas in the pyramidal layer of the human hippocampus. (c) Cajal's drawing of fi brous astrocytes of human cerebral cortex surrounding a blood vessel. Reproduced from (145).
Fig. 2
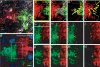
Astrocytic domain organization varies with pathology. The domain organization of protoplasmic astrocytes is lost in epileptic brains, but maintained in neurodegeneration. (a) Reactive astrocytes 1 week post-iron injection lose the domain organization. Diolistic labelling of the cortex of a GFAP-GFP mouse 1 week post-iron injection near injection site. Two adjacent GFP positive astrocytes are labeled with DiI and DiD. DAPI, blue, GFP, green, DiI, red, DiD, white. (b–e) High power of yellow box in (a). area of overlap delineated in grey, red line is border of the domain of the red cell, green line is the border of the domain of the white cell. (g–h) Yellow lines indicate the processes of the cell that pass into the domain of the adjacent cell's domain represented by the dotted line. (f) Cortical astrocytes in an Alzheimer disease model Tg2576 become reactive, but do not lose the domain organization. Diolistic labelling of cortical astrocytes in Tg2576 mouse. (g–j) High power of blue box in (f) showing limited overlap between adjacent cells. (k–n) Adjacent control astrocytes demonstrating the domain organization. Scale: (a) 20 μ m; (g–h) 10 μ m. From (22).
Fig. 3
Astrocytic expression of glutamate transporters varies in different areas of the CNS. Double-transgenic mice expressing fl orescent proteins under the GLAST and GLT-1 promoters were used to study the expression of GLAST and GLT-1 during development of the CNS. All images are sagittal sections from GLAST–DsRed/GLT-1–eGFP double-transgenic mice. (a) Composite fluorescent image showing the expression of DsRed (GLAST) (red) and eGFP (GLT-1) (green) in the brain at P1. (b) Composite image showing the expression of DsRed (GLAST) and eGFP (GLT-1) in the P24 brain. (c–e) Fluorescent images of the cerebellum at P1 (c) and P24 (d, e). Both GLAST and GLT-1 promoters were active in Bergmann glia (Bg) (yellow arrows in (e)), although neither promoter was active in Purkinje neurons (white arrows in (e)). (f–h) Fluorescent images of the cortex from P1 (f) and P24 (g, h). Layer 4 is shown at higher magnification in (h). (i–k) Fluorescent images of the hippocampus from a P1 (i) or P24 (j, k). Both DsRed (GLAST) and eGFP (GLT-1) were expressed by radial glia in the dentate gyrus (yellow arrows in (k)). (l–n) Fluorescent images of spinal cord from a P1 (l) or P24 (m, n). gm gray matter; wm white matter. A region of the ventral white matter is shown at a higher magnification in (n). Scale: (a–b) 2 mm; (c, d) 300 μ m; (e) 50 μ m; (f, g) 300 μ m; (h) 50 μ m; (i, j), 300 μ m; (k) 50 μ m; (l, m) 300 μ m; (n) 50 μ m. From (55).
Fig. 4
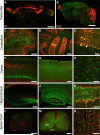
Four major classes of GFAP+ cells coreside within the human neocortex. Human brains were immunolabeled with GFAP and analyzed throughout all layers of the cortex to determine subclasses of human astrocytes. Layer 1 is composed of the cell bodies of interlaminar astrocytes, whose processes extend over millimetre lengths through layers 2–4 and are characterized by their tortuous morphology. Protoplasmic astrocytes, the most common, reside in layers 2–6. Polarized astrocytes are found only in humans and are seen sparsely in layers 5–6. They extend millimetre-long processes that are characterized by varicosities. Fibrous astrocytes are found in the white matter and contain numerous overlapping processes.Yellow lines indicate areas in which the different classes of astrocytes reside. Scale = 150 μm. Reproduced from (8).
Fig. 5
Hominid-specific astrocytic phenotypes pervade the human brain. (a) Varicose projection astrocytes reside in layers 5–6 and extend long processes characterized by evenly spaced varicosities. GFAP,white, MAP2,red, DAPI,blue.Yellow arrowheads indicate numerous long processes. (b) Pial surface and layers 1–2 of human cortex. GFAP,white, DAPI,blue.Yellow line indicates border between layers I and II. (c) Process from a varicose projection astrocyte. GFAP,white. (d) Interlaminar astrocyte processes characterized by their tortuousity. GFAP,white. Scale: (a,b) 100 μm; (c,d) = 10 μm. Reproduced from (8).
Fig. 6
Human astrocytes are larger and more complex than rodent and other primates. Mouse, Rhesus Monkey, and Human astrocytes are compared by GFAP staining (white). Scale = 20 μm.
Similar articles
- Astrocytes: Glutamate transport and alternate splicing of transporters.
Lee A, Pow DV. Lee A, et al. Int J Biochem Cell Biol. 2010 Dec;42(12):1901-6. doi: 10.1016/j.biocel.2010.09.016. Epub 2010 Sep 29. Int J Biochem Cell Biol. 2010. PMID: 20883814 Review. - Do Evolutionary Changes in Astrocytes Contribute to the Computational Power of the Hominid Brain?
Oberheim Bush NA, Nedergaard M. Oberheim Bush NA, et al. Neurochem Res. 2017 Sep;42(9):2577-2587. doi: 10.1007/s11064-017-2363-0. Epub 2017 Aug 19. Neurochem Res. 2017. PMID: 28822066 Review. - The neurobiology of glia in the context of water and ion homeostasis.
Simard M, Nedergaard M. Simard M, et al. Neuroscience. 2004;129(4):877-96. doi: 10.1016/j.neuroscience.2004.09.053. Neuroscience. 2004. PMID: 15561405 Review. - Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2.
Hinson SR, Roemer SF, Lucchinetti CF, Fryer JP, Kryzer TJ, Chamberlain JL, Howe CL, Pittock SJ, Lennon VA. Hinson SR, et al. J Exp Med. 2008 Oct 27;205(11):2473-81. doi: 10.1084/jem.20081241. Epub 2008 Oct 6. J Exp Med. 2008. PMID: 18838545 Free PMC article. - A glutamatergic component of lead toxicity in adult brain: the role of astrocytic glutamate transporters.
Struzyńska L. Struzyńska L. Neurochem Int. 2009 Jul-Aug;55(1-3):151-6. doi: 10.1016/j.neuint.2009.01.025. Epub 2009 Feb 13. Neurochem Int. 2009. PMID: 19428820 Review.
Cited by
- Honey Enriched with Additives Alleviates Behavioral, Oxidative Stress, and Brain Alterations Induced by Heavy Metals and Imidacloprid in Zebrafish.
Paduraru E, Jijie R, Simionov IA, Gavrilescu CM, Ilie T, Iacob D, Lupitu A, Moisa C, Muresan C, Copolovici L, Copolovici DM, Mihalache G, Lipsa FD, Solcan G, Danelet GA, Nicoara M, Ciobica A, Solcan C. Paduraru E, et al. Int J Mol Sci. 2024 Oct 31;25(21):11730. doi: 10.3390/ijms252111730. Int J Mol Sci. 2024. PMID: 39519279 Free PMC article. - Computational modeling of the relationship between morphological heterogeneity and functional responses in mouse hippocampal astrocytes.
Freund A, Mayr A, Winkler P, Weber R, Tervonen A, Refaeli R, Lenk K. Freund A, et al. Front Cell Neurosci. 2024 Oct 17;18:1474948. doi: 10.3389/fncel.2024.1474948. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39484184 Free PMC article. - Of potential new treatment targets and polythetic approach in meningoencephalitis of unknown origin: a review.
Nessler JN, Tipold A. Nessler JN, et al. Front Vet Sci. 2024 Oct 15;11:1465689. doi: 10.3389/fvets.2024.1465689. eCollection 2024. Front Vet Sci. 2024. PMID: 39474275 Free PMC article. Review. - Mechanisms and Therapeutic Prospects of Microglia-Astrocyte Interactions in Neuropathic Pain Following Spinal Cord Injury.
Liu Y, Cai X, Shi B, Mo Y, Zhang J, Luo W, Yu B, Li X. Liu Y, et al. Mol Neurobiol. 2024 Oct 29. doi: 10.1007/s12035-024-04562-1. Online ahead of print. Mol Neurobiol. 2024. PMID: 39470872 Review. - Sex Differences in Astrocyte Activity.
Gozlan E, Lewit-Cohen Y, Frenkel D. Gozlan E, et al. Cells. 2024 Oct 18;13(20):1724. doi: 10.3390/cells13201724. Cells. 2024. PMID: 39451242 Free PMC article. Review.
References
- Virchow RLK. Die cellularpathologie in ihrer begründung auf physiologische und pathologische gewebelehre. A. Hirschwald; Berlin: 1858. - PubMed
- Golgi C. Contribuzione alla fina Anatomia degli organi centrali del sistema nervosos. Rivista clinica di Bologna; Bologna: 1871.
- Lenhossek M. Der feinere Bau des Nervensystems im Lichte neuester Forschung. Fischer's Medicinische Buchhandlung; Berlin: 1893.
- Kölliker A. Handbuch der gewebelehre des menschen. 1889. p. 6. umgearb. aufl. ed., n.p.
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS029813/NS/NINDS NIH HHS/United States
- R01 NS078167/NS/NINDS NIH HHS/United States
- R01 NS052534/NS/NINDS NIH HHS/United States
- R01 NS075177/NS/NINDS NIH HHS/United States
- R01 NS038073/NS/NINDS NIH HHS/United States
- R01 NS039559/NS/NINDS NIH HHS/United States
- R01 NS056188/NS/NINDS NIH HHS/United States
- R37 NS029813/NS/NINDS NIH HHS/United States
- P01 NS050315/NS/NINDS NIH HHS/United States
- R01 NS075345/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources