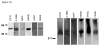Soluble multi-trimeric TNF superfamily ligand adjuvants enhance immune responses to a HIV-1 Gag DNA vaccine - PubMed (original) (raw)
Soluble multi-trimeric TNF superfamily ligand adjuvants enhance immune responses to a HIV-1 Gag DNA vaccine
Saravana K Kanagavelu et al. Vaccine. 2012.
Abstract
Background: DNA vaccines remain an important component of HIV vaccination strategies, typically as part of a prime/boost vaccination strategy with viral vector or protein boost. A number of DNA prime/viral vector boost vaccines are currently being evaluated for both preclinical studies and in Phase I and Phase II clinical trials. These vaccines would benefit from molecular adjuvants that increase correlates of immunity during the DNA prime. While HIV vaccine immune correlates are still not well defined, there are a number of immune assays that have been shown to correlate with protection from viral challenge including CD8+ T cell avidity, antigen-specific proliferation, and polyfunctional cytokine secretion.
Methodology and principal findings: Recombinant DNA vaccine adjuvants composed of a fusion between Surfactant Protein D (SP-D) and either CD40 Ligand (CD40L) or GITR Ligand (GITRL) were previously shown to enhance HIV-1 Gag DNA vaccines. Here we show that similar fusion constructs composed of the TNF superfamily ligands (TNFSFL) 4-1BBL, OX40L, RANKL, LIGHT, CD70, and BAFF can also enhanced immune responses to a HIV-1 Gag DNA vaccine. BALB/c mice were vaccinated intramuscularly with plasmids expressing secreted Gag and SP-D-TNFSFL fusions. Initially, mice were analyzed 2 weeks or 7 weeks following vaccination to evaluate the relative efficacy of each SP-D-TNFSFL construct. All SP-D-TNFSFL constructs enhanced at least one Gag-specific immune response compared to the parent vaccine. Importantly, the constructs SP-D-4-1BBL, SP-D-OX40L, and SP-D-LIGHT enhanced CD8+ T cell avidity and CD8+/CD4+ T cell proliferation 7 weeks post vaccination. These avidity and proliferation data suggest that 4-1BBL, OX40L, and LIGHT fusion constructs may be particularly effective as vaccine adjuvants. Constructs SP-D-OX40L, SP-D-LIGHT, and SP-D-BAFF enhanced Gag-specific IL-2 secretion in memory T cells, suggesting these adjuvants can increase the number of self-renewing Gag-specific CD8+ and/or CD4+ T cells. Finally adjuvants SP-D-OX40L and SP-D-CD70 increased T(H)1 (IgG2a) but not T(H)2 (IgG1) antibody responses in the vaccinated animals. Surprisingly, the B cell-activating protein BAFF did not enhance anti-Gag antibody responses when given as an SP-D fusion adjuvant, but nonetheless enhanced CD4+ and CD8+ T cell responses.
Conclusions: We present evidence that various SP-D-TNFSFL fusion constructs can enhance immune responses following DNA vaccination with HIV-1 Gag expression plasmid. These data support the continued evaluation of SP-D-TNFSFL fusion proteins as molecular adjuvants for DNA and/or viral vector vaccines. Constructs of particular interest included SP-D-OX40L, SP-D-4-1BBL, SP-D-LIGHT, and SP-D-CD70. SP-D-BAFF was surprisingly effective at enhancing T cell responses, despite its inability to enhance anti-Gag antibody secretion.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
Figure 1. Expression and activity of murine SP-D-TNFSF ligands in vitro
(A) 293T cells were transfected with DNA plasmid expression vectors encoding various SP-D-TNFSF ligand fusion proteins. After 48 hour culture, supernatant was collected and run on an SDS-PAGE gel in the presence of reducing agent. Western blots were performed using polyclonal antibody to murine OX40L, BAFF, LIGHT, RANKL, CD27L AND 4-1BBL proteins (Left Panel). To confirm the presence of multi-trimer complexes a Western blot was performed on the SP-D-TNFSFL as above but using non-denaturing PAGE in the absence of reducing agent (Right Panel). (B) Costimulation of CD4+ T cells with anti-CD3 antibody and supernatant from 293T cells transfected with the various SP-D-TNFSF ligand plasmids. An equal amount of each SP-D-TNFSFL protein was added based on ELISA assay. Transfected supernatants were mixed with control pcDNA3.1 supernatant so that each well received the same total volume of 293T supernatant. Representative data from two independent experiments is shown. Error bars represent multiple tests within the same experiment. Supernatant from SP-D-CD40L, SP-D-CD70, SP-D-GITRL, SP-D-LIGHT, SP-D-41BBL, and SP-D-RANKL induced a significant increase in the proliferation of CD4+ T cells. (* p<0.05; ** p<0.01; *** p<0.001 by Student’s t test compared to pcDNA3.1 transfected 293T cell supernatant control). (C) In vitro activity of pSP-D-CD40L using an SEAP CD40 reporter system. An equivalent amount of 293T supernatant from pcDNA3.1 or pSP-D-CD40L was incubated with 293-CD40-SEAP NF-κB reporter cells. Activity was dose dependent in response to dilution of the supernatant. (D) In vivo activity of pSP-D-BAFF. C57BL/6 mice (3 mice per group) were injected intramuscularly with 100 ug of either pcDNA3.1 or pSP-D-BAFF plasmid. SP-D-BAFF significantly increased the total number of B220+ B cells in both spleen and lymph node of animals. * p<0.05, ** p<0.01 by Student’s t test compared to control mice.
Figure 1. Expression and activity of murine SP-D-TNFSF ligands in vitro
(A) 293T cells were transfected with DNA plasmid expression vectors encoding various SP-D-TNFSF ligand fusion proteins. After 48 hour culture, supernatant was collected and run on an SDS-PAGE gel in the presence of reducing agent. Western blots were performed using polyclonal antibody to murine OX40L, BAFF, LIGHT, RANKL, CD27L AND 4-1BBL proteins (Left Panel). To confirm the presence of multi-trimer complexes a Western blot was performed on the SP-D-TNFSFL as above but using non-denaturing PAGE in the absence of reducing agent (Right Panel). (B) Costimulation of CD4+ T cells with anti-CD3 antibody and supernatant from 293T cells transfected with the various SP-D-TNFSF ligand plasmids. An equal amount of each SP-D-TNFSFL protein was added based on ELISA assay. Transfected supernatants were mixed with control pcDNA3.1 supernatant so that each well received the same total volume of 293T supernatant. Representative data from two independent experiments is shown. Error bars represent multiple tests within the same experiment. Supernatant from SP-D-CD40L, SP-D-CD70, SP-D-GITRL, SP-D-LIGHT, SP-D-41BBL, and SP-D-RANKL induced a significant increase in the proliferation of CD4+ T cells. (* p<0.05; ** p<0.01; *** p<0.001 by Student’s t test compared to pcDNA3.1 transfected 293T cell supernatant control). (C) In vitro activity of pSP-D-CD40L using an SEAP CD40 reporter system. An equivalent amount of 293T supernatant from pcDNA3.1 or pSP-D-CD40L was incubated with 293-CD40-SEAP NF-κB reporter cells. Activity was dose dependent in response to dilution of the supernatant. (D) In vivo activity of pSP-D-BAFF. C57BL/6 mice (3 mice per group) were injected intramuscularly with 100 ug of either pcDNA3.1 or pSP-D-BAFF plasmid. SP-D-BAFF significantly increased the total number of B220+ B cells in both spleen and lymph node of animals. * p<0.05, ** p<0.01 by Student’s t test compared to control mice.
Figure 1. Expression and activity of murine SP-D-TNFSF ligands in vitro
(A) 293T cells were transfected with DNA plasmid expression vectors encoding various SP-D-TNFSF ligand fusion proteins. After 48 hour culture, supernatant was collected and run on an SDS-PAGE gel in the presence of reducing agent. Western blots were performed using polyclonal antibody to murine OX40L, BAFF, LIGHT, RANKL, CD27L AND 4-1BBL proteins (Left Panel). To confirm the presence of multi-trimer complexes a Western blot was performed on the SP-D-TNFSFL as above but using non-denaturing PAGE in the absence of reducing agent (Right Panel). (B) Costimulation of CD4+ T cells with anti-CD3 antibody and supernatant from 293T cells transfected with the various SP-D-TNFSF ligand plasmids. An equal amount of each SP-D-TNFSFL protein was added based on ELISA assay. Transfected supernatants were mixed with control pcDNA3.1 supernatant so that each well received the same total volume of 293T supernatant. Representative data from two independent experiments is shown. Error bars represent multiple tests within the same experiment. Supernatant from SP-D-CD40L, SP-D-CD70, SP-D-GITRL, SP-D-LIGHT, SP-D-41BBL, and SP-D-RANKL induced a significant increase in the proliferation of CD4+ T cells. (* p<0.05; ** p<0.01; *** p<0.001 by Student’s t test compared to pcDNA3.1 transfected 293T cell supernatant control). (C) In vitro activity of pSP-D-CD40L using an SEAP CD40 reporter system. An equivalent amount of 293T supernatant from pcDNA3.1 or pSP-D-CD40L was incubated with 293-CD40-SEAP NF-κB reporter cells. Activity was dose dependent in response to dilution of the supernatant. (D) In vivo activity of pSP-D-BAFF. C57BL/6 mice (3 mice per group) were injected intramuscularly with 100 ug of either pcDNA3.1 or pSP-D-BAFF plasmid. SP-D-BAFF significantly increased the total number of B220+ B cells in both spleen and lymph node of animals. * p<0.05, ** p<0.01 by Student’s t test compared to control mice.
Figure 1. Expression and activity of murine SP-D-TNFSF ligands in vitro
(A) 293T cells were transfected with DNA plasmid expression vectors encoding various SP-D-TNFSF ligand fusion proteins. After 48 hour culture, supernatant was collected and run on an SDS-PAGE gel in the presence of reducing agent. Western blots were performed using polyclonal antibody to murine OX40L, BAFF, LIGHT, RANKL, CD27L AND 4-1BBL proteins (Left Panel). To confirm the presence of multi-trimer complexes a Western blot was performed on the SP-D-TNFSFL as above but using non-denaturing PAGE in the absence of reducing agent (Right Panel). (B) Costimulation of CD4+ T cells with anti-CD3 antibody and supernatant from 293T cells transfected with the various SP-D-TNFSF ligand plasmids. An equal amount of each SP-D-TNFSFL protein was added based on ELISA assay. Transfected supernatants were mixed with control pcDNA3.1 supernatant so that each well received the same total volume of 293T supernatant. Representative data from two independent experiments is shown. Error bars represent multiple tests within the same experiment. Supernatant from SP-D-CD40L, SP-D-CD70, SP-D-GITRL, SP-D-LIGHT, SP-D-41BBL, and SP-D-RANKL induced a significant increase in the proliferation of CD4+ T cells. (* p<0.05; ** p<0.01; *** p<0.001 by Student’s t test compared to pcDNA3.1 transfected 293T cell supernatant control). (C) In vitro activity of pSP-D-CD40L using an SEAP CD40 reporter system. An equivalent amount of 293T supernatant from pcDNA3.1 or pSP-D-CD40L was incubated with 293-CD40-SEAP NF-κB reporter cells. Activity was dose dependent in response to dilution of the supernatant. (D) In vivo activity of pSP-D-BAFF. C57BL/6 mice (3 mice per group) were injected intramuscularly with 100 ug of either pcDNA3.1 or pSP-D-BAFF plasmid. SP-D-BAFF significantly increased the total number of B220+ B cells in both spleen and lymph node of animals. * p<0.05, ** p<0.01 by Student’s t test compared to control mice.
Figure 2. Comparison of in vivo immune responses to SP-D-TNFSFL adjuvants
(A) Immunization schedule. BALB/c mice (5 per group) were immunized intramuscularly with pscGag plasmid either alone or in combination with SP-D-TNFSFL adjuvants on days 0, 14, and 28. A total of 100ug of plasmid was injected (80ug pscGag + 20ug SP-D-TNFSFL or pcDNA3.1), 50ug into each quadriceps muscle. Two weeks later mice were sacrificed and splenocytes analyzed for Gag specific immune responses. (B) IFN-γ ELISPOT assay to measure Gag specific CD8+ T cell responses. Splenocytes were collected and cultured for 18 h in the presence of 10ug/ml HIV-1 Gag CD8+ specific peptide AMQMLKETI. The adjuvants SP-D-CD40L, SP-D-GITRL, SP-D-4-1BBL, SP-D-LIGHT, and SP-D-CD70 significantly increased Gag-specific IFN-γ secretion compared to pscGag + pcDNA3.1 vaccination. (C) Induction of Gag-specific proliferation. Splenocytes were cultured for 5 days in the presence of Gag protein. Cultures were pulsed with 3[H]-thymidine overnight before cell harvesting onto glass filters and scintillation counting to calculate stimulation index. The adjuvants SP-D-CD40L and SP-D-GITRL induced a significant increase in stimulation index compared to pscGag + pcDNA3.1. (D) IgG antibody responses to Gag DNA vaccination. Total IgG specific for Gag was measured by ELISA assay from mouse serum collected on day 42. The adjuvant SP-D-LIGHT showed increased absorbance compared to pscGag + pcDNA3.1, but did not reach statistical significance. Adjuvants SP-D-OX40L and SP-D-RANKL reduced total IgG levels. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag + pcDNA3.1 vaccination.
Figure 2. Comparison of in vivo immune responses to SP-D-TNFSFL adjuvants
(A) Immunization schedule. BALB/c mice (5 per group) were immunized intramuscularly with pscGag plasmid either alone or in combination with SP-D-TNFSFL adjuvants on days 0, 14, and 28. A total of 100ug of plasmid was injected (80ug pscGag + 20ug SP-D-TNFSFL or pcDNA3.1), 50ug into each quadriceps muscle. Two weeks later mice were sacrificed and splenocytes analyzed for Gag specific immune responses. (B) IFN-γ ELISPOT assay to measure Gag specific CD8+ T cell responses. Splenocytes were collected and cultured for 18 h in the presence of 10ug/ml HIV-1 Gag CD8+ specific peptide AMQMLKETI. The adjuvants SP-D-CD40L, SP-D-GITRL, SP-D-4-1BBL, SP-D-LIGHT, and SP-D-CD70 significantly increased Gag-specific IFN-γ secretion compared to pscGag + pcDNA3.1 vaccination. (C) Induction of Gag-specific proliferation. Splenocytes were cultured for 5 days in the presence of Gag protein. Cultures were pulsed with 3[H]-thymidine overnight before cell harvesting onto glass filters and scintillation counting to calculate stimulation index. The adjuvants SP-D-CD40L and SP-D-GITRL induced a significant increase in stimulation index compared to pscGag + pcDNA3.1. (D) IgG antibody responses to Gag DNA vaccination. Total IgG specific for Gag was measured by ELISA assay from mouse serum collected on day 42. The adjuvant SP-D-LIGHT showed increased absorbance compared to pscGag + pcDNA3.1, but did not reach statistical significance. Adjuvants SP-D-OX40L and SP-D-RANKL reduced total IgG levels. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag + pcDNA3.1 vaccination.
Figure 2. Comparison of in vivo immune responses to SP-D-TNFSFL adjuvants
(A) Immunization schedule. BALB/c mice (5 per group) were immunized intramuscularly with pscGag plasmid either alone or in combination with SP-D-TNFSFL adjuvants on days 0, 14, and 28. A total of 100ug of plasmid was injected (80ug pscGag + 20ug SP-D-TNFSFL or pcDNA3.1), 50ug into each quadriceps muscle. Two weeks later mice were sacrificed and splenocytes analyzed for Gag specific immune responses. (B) IFN-γ ELISPOT assay to measure Gag specific CD8+ T cell responses. Splenocytes were collected and cultured for 18 h in the presence of 10ug/ml HIV-1 Gag CD8+ specific peptide AMQMLKETI. The adjuvants SP-D-CD40L, SP-D-GITRL, SP-D-4-1BBL, SP-D-LIGHT, and SP-D-CD70 significantly increased Gag-specific IFN-γ secretion compared to pscGag + pcDNA3.1 vaccination. (C) Induction of Gag-specific proliferation. Splenocytes were cultured for 5 days in the presence of Gag protein. Cultures were pulsed with 3[H]-thymidine overnight before cell harvesting onto glass filters and scintillation counting to calculate stimulation index. The adjuvants SP-D-CD40L and SP-D-GITRL induced a significant increase in stimulation index compared to pscGag + pcDNA3.1. (D) IgG antibody responses to Gag DNA vaccination. Total IgG specific for Gag was measured by ELISA assay from mouse serum collected on day 42. The adjuvant SP-D-LIGHT showed increased absorbance compared to pscGag + pcDNA3.1, but did not reach statistical significance. Adjuvants SP-D-OX40L and SP-D-RANKL reduced total IgG levels. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag + pcDNA3.1 vaccination.
Figure 2. Comparison of in vivo immune responses to SP-D-TNFSFL adjuvants
(A) Immunization schedule. BALB/c mice (5 per group) were immunized intramuscularly with pscGag plasmid either alone or in combination with SP-D-TNFSFL adjuvants on days 0, 14, and 28. A total of 100ug of plasmid was injected (80ug pscGag + 20ug SP-D-TNFSFL or pcDNA3.1), 50ug into each quadriceps muscle. Two weeks later mice were sacrificed and splenocytes analyzed for Gag specific immune responses. (B) IFN-γ ELISPOT assay to measure Gag specific CD8+ T cell responses. Splenocytes were collected and cultured for 18 h in the presence of 10ug/ml HIV-1 Gag CD8+ specific peptide AMQMLKETI. The adjuvants SP-D-CD40L, SP-D-GITRL, SP-D-4-1BBL, SP-D-LIGHT, and SP-D-CD70 significantly increased Gag-specific IFN-γ secretion compared to pscGag + pcDNA3.1 vaccination. (C) Induction of Gag-specific proliferation. Splenocytes were cultured for 5 days in the presence of Gag protein. Cultures were pulsed with 3[H]-thymidine overnight before cell harvesting onto glass filters and scintillation counting to calculate stimulation index. The adjuvants SP-D-CD40L and SP-D-GITRL induced a significant increase in stimulation index compared to pscGag + pcDNA3.1. (D) IgG antibody responses to Gag DNA vaccination. Total IgG specific for Gag was measured by ELISA assay from mouse serum collected on day 42. The adjuvant SP-D-LIGHT showed increased absorbance compared to pscGag + pcDNA3.1, but did not reach statistical significance. Adjuvants SP-D-OX40L and SP-D-RANKL reduced total IgG levels. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag + pcDNA3.1 vaccination.
Figure 3. Adjuvants SP-D-4-1BBL, SP-D-OX40L, SP-D-LIGHT, and SP-D-CD40L enhance memory CD8+ T cell avidity to Gag
(A) Immunization schedule. BALB/c mice were immunized intramuscularly with pscGag plasmid either with pcDNA3.1 or SP-D-TNFSFL adjuvants on day 0, 14 and 28. Mice were sacrificed on day 77 and splenocytes analyzed for Gag-specific memory T cell responses. (B) and (C) Peptide dilution ELISPOT assay. Splenocytes were cultured with serial dilutions of CD8+ T cell specific peptide AMQMLKETI for 18 hours. At 1ug/ml plasmid, all adjuvants with the exception of SP-D-CD70 induced a greater IFN-γ ELISPOT response compared to pscGag. Adjuvants SP-D-4-1BBL, SP-D-OX40L, SP-D-LIGHT, and SP-D-CD40L also induced a greater ELISPOT response at 1ng/ml peptide compared to pscGag + pcDNA3.1. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag vaccination.
Figure 3. Adjuvants SP-D-4-1BBL, SP-D-OX40L, SP-D-LIGHT, and SP-D-CD40L enhance memory CD8+ T cell avidity to Gag
(A) Immunization schedule. BALB/c mice were immunized intramuscularly with pscGag plasmid either with pcDNA3.1 or SP-D-TNFSFL adjuvants on day 0, 14 and 28. Mice were sacrificed on day 77 and splenocytes analyzed for Gag-specific memory T cell responses. (B) and (C) Peptide dilution ELISPOT assay. Splenocytes were cultured with serial dilutions of CD8+ T cell specific peptide AMQMLKETI for 18 hours. At 1ug/ml plasmid, all adjuvants with the exception of SP-D-CD70 induced a greater IFN-γ ELISPOT response compared to pscGag. Adjuvants SP-D-4-1BBL, SP-D-OX40L, SP-D-LIGHT, and SP-D-CD40L also induced a greater ELISPOT response at 1ng/ml peptide compared to pscGag + pcDNA3.1. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag vaccination.
Figure 3. Adjuvants SP-D-4-1BBL, SP-D-OX40L, SP-D-LIGHT, and SP-D-CD40L enhance memory CD8+ T cell avidity to Gag
(A) Immunization schedule. BALB/c mice were immunized intramuscularly with pscGag plasmid either with pcDNA3.1 or SP-D-TNFSFL adjuvants on day 0, 14 and 28. Mice were sacrificed on day 77 and splenocytes analyzed for Gag-specific memory T cell responses. (B) and (C) Peptide dilution ELISPOT assay. Splenocytes were cultured with serial dilutions of CD8+ T cell specific peptide AMQMLKETI for 18 hours. At 1ug/ml plasmid, all adjuvants with the exception of SP-D-CD70 induced a greater IFN-γ ELISPOT response compared to pscGag. Adjuvants SP-D-4-1BBL, SP-D-OX40L, SP-D-LIGHT, and SP-D-CD40L also induced a greater ELISPOT response at 1ng/ml peptide compared to pscGag + pcDNA3.1. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag vaccination.
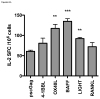
Figure 4. Gag-specific IL-2 secretion by memory T cells
(A) ELISPOT assay measuring the number of Gag specific CD8+ T cells secreting IL-2 in response to AMQMLKETI peptide. BALB/c mice (n=5) were immunized with HIV-1 Gag with SP-D-TNFSFL plasmids or empty vector pcDNA3.1, as indicated. Seven weeks after the third vaccination, mice splenocytes from each group were pooled and assayed in triplicate. SP-D-OX40L, SP-D-BAFF, and SP-D-LIGHT induced a significant increase in CD8+ T cell IL-2 secretion. (B) ELISPOT assay for SP-D-CD40L and SP-D-CD70 adjuvants. Construct SP-D-CD40L increased IL-2 secretion from CD8+ T cells. (C) IL-2 secretion during incubation of splenocytes with whole Gag protein. To evaluate both CD8+ and CD4+ T cell responses, splenocytes were incubated with Gag p55 protein. SP-D-LIGHT and SP-D-RANKL displayed enhanced IL-2 secretion using protein for stimulation. (D) Whole Gag ELISPOT for SP-D-CD40L and SP-D-CD70. These two adjuvants did not increase IL-2 secretion when using Gag protein to at both CD4+ and CD8+ T cell responses. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag + pcDNA3.1 vaccination.
Figure 4. Gag-specific IL-2 secretion by memory T cells
(A) ELISPOT assay measuring the number of Gag specific CD8+ T cells secreting IL-2 in response to AMQMLKETI peptide. BALB/c mice (n=5) were immunized with HIV-1 Gag with SP-D-TNFSFL plasmids or empty vector pcDNA3.1, as indicated. Seven weeks after the third vaccination, mice splenocytes from each group were pooled and assayed in triplicate. SP-D-OX40L, SP-D-BAFF, and SP-D-LIGHT induced a significant increase in CD8+ T cell IL-2 secretion. (B) ELISPOT assay for SP-D-CD40L and SP-D-CD70 adjuvants. Construct SP-D-CD40L increased IL-2 secretion from CD8+ T cells. (C) IL-2 secretion during incubation of splenocytes with whole Gag protein. To evaluate both CD8+ and CD4+ T cell responses, splenocytes were incubated with Gag p55 protein. SP-D-LIGHT and SP-D-RANKL displayed enhanced IL-2 secretion using protein for stimulation. (D) Whole Gag ELISPOT for SP-D-CD40L and SP-D-CD70. These two adjuvants did not increase IL-2 secretion when using Gag protein to at both CD4+ and CD8+ T cell responses. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag + pcDNA3.1 vaccination.
Figure 4. Gag-specific IL-2 secretion by memory T cells
(A) ELISPOT assay measuring the number of Gag specific CD8+ T cells secreting IL-2 in response to AMQMLKETI peptide. BALB/c mice (n=5) were immunized with HIV-1 Gag with SP-D-TNFSFL plasmids or empty vector pcDNA3.1, as indicated. Seven weeks after the third vaccination, mice splenocytes from each group were pooled and assayed in triplicate. SP-D-OX40L, SP-D-BAFF, and SP-D-LIGHT induced a significant increase in CD8+ T cell IL-2 secretion. (B) ELISPOT assay for SP-D-CD40L and SP-D-CD70 adjuvants. Construct SP-D-CD40L increased IL-2 secretion from CD8+ T cells. (C) IL-2 secretion during incubation of splenocytes with whole Gag protein. To evaluate both CD8+ and CD4+ T cell responses, splenocytes were incubated with Gag p55 protein. SP-D-LIGHT and SP-D-RANKL displayed enhanced IL-2 secretion using protein for stimulation. (D) Whole Gag ELISPOT for SP-D-CD40L and SP-D-CD70. These two adjuvants did not increase IL-2 secretion when using Gag protein to at both CD4+ and CD8+ T cell responses. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag + pcDNA3.1 vaccination.
Figure 4. Gag-specific IL-2 secretion by memory T cells
(A) ELISPOT assay measuring the number of Gag specific CD8+ T cells secreting IL-2 in response to AMQMLKETI peptide. BALB/c mice (n=5) were immunized with HIV-1 Gag with SP-D-TNFSFL plasmids or empty vector pcDNA3.1, as indicated. Seven weeks after the third vaccination, mice splenocytes from each group were pooled and assayed in triplicate. SP-D-OX40L, SP-D-BAFF, and SP-D-LIGHT induced a significant increase in CD8+ T cell IL-2 secretion. (B) ELISPOT assay for SP-D-CD40L and SP-D-CD70 adjuvants. Construct SP-D-CD40L increased IL-2 secretion from CD8+ T cells. (C) IL-2 secretion during incubation of splenocytes with whole Gag protein. To evaluate both CD8+ and CD4+ T cell responses, splenocytes were incubated with Gag p55 protein. SP-D-LIGHT and SP-D-RANKL displayed enhanced IL-2 secretion using protein for stimulation. (D) Whole Gag ELISPOT for SP-D-CD40L and SP-D-CD70. These two adjuvants did not increase IL-2 secretion when using Gag protein to at both CD4+ and CD8+ T cell responses. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag + pcDNA3.1 vaccination.
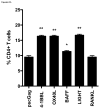
Figure 5. CD4+ and CD8+ T cell memory proliferation responses induced by SP-D-TNFSFL adjuvants
BALB/c mice (n=5) were immunized with HIV-1 Gag plus empty vector pcDNA3.1 or SP-D-TNFSFL plasmids, as indicated. Seven weeks after the third vaccination, mice splenocytes from each group were pooled, labeled with CFSE, and cultured with p55 Gag protein for 5 days. Cells were fluorescently labeled with anti-mouse CD3, CD4, and CD8 antibodies to differentiate CD4+ and CD8+ T cell populations. Percent proliferation was measured as the ratio of total CFSElow cells to non-proliferating CFSEhi cells. Samples were pooled and assayed in triplicate. (A) Significant CD4+ T cell proliferation was observed in the presence of SP-D-4-1BBL, SP-D-OX40L, SP-D-BAFF, and SP-D-LIGHT when compared to pscGag + pcDNA3.1. (B) CD8+ T cell proliferation was induced by SP-D-4-1BBL, SP-D-OX40L, and SP-D-BAFF. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag + pcDNA3.1 vaccination.
Figure 5. CD4+ and CD8+ T cell memory proliferation responses induced by SP-D-TNFSFL adjuvants
BALB/c mice (n=5) were immunized with HIV-1 Gag plus empty vector pcDNA3.1 or SP-D-TNFSFL plasmids, as indicated. Seven weeks after the third vaccination, mice splenocytes from each group were pooled, labeled with CFSE, and cultured with p55 Gag protein for 5 days. Cells were fluorescently labeled with anti-mouse CD3, CD4, and CD8 antibodies to differentiate CD4+ and CD8+ T cell populations. Percent proliferation was measured as the ratio of total CFSElow cells to non-proliferating CFSEhi cells. Samples were pooled and assayed in triplicate. (A) Significant CD4+ T cell proliferation was observed in the presence of SP-D-4-1BBL, SP-D-OX40L, SP-D-BAFF, and SP-D-LIGHT when compared to pscGag + pcDNA3.1. (B) CD8+ T cell proliferation was induced by SP-D-4-1BBL, SP-D-OX40L, and SP-D-BAFF. * p<0.05; ** p<0.01; *** p<0.001 compared to pscGag + pcDNA3.1 vaccination.
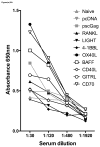
Figure 6. SP-D-TNFSFL adjuvants increase IgG2a antibody responses to Gag
Mice were immunized with HIV-1 Gag alone or combination of SP-D-TNFSFL plasmids, as indicated. Seven weeks after the third vaccination, mice were bled and serum from each group was serially diluted and assayed for IgG binding to p55 Gag antigen. Total IgG, IgG1, and IgG2a titers were measured by ELISA. (A) Total IgG response. (B) IgG2a response. (C) IgG1 response. SP-D-CD70, SP-D-OX40L, and SP-D-BAFF enhanced total IgG binding at 1:30 and 1:120 dilutions. SP-D-CD70 and SP-D-OX40L enhanced IgG2a binding at titers as low as 1:480.
Figure 6. SP-D-TNFSFL adjuvants increase IgG2a antibody responses to Gag
Mice were immunized with HIV-1 Gag alone or combination of SP-D-TNFSFL plasmids, as indicated. Seven weeks after the third vaccination, mice were bled and serum from each group was serially diluted and assayed for IgG binding to p55 Gag antigen. Total IgG, IgG1, and IgG2a titers were measured by ELISA. (A) Total IgG response. (B) IgG2a response. (C) IgG1 response. SP-D-CD70, SP-D-OX40L, and SP-D-BAFF enhanced total IgG binding at 1:30 and 1:120 dilutions. SP-D-CD70 and SP-D-OX40L enhanced IgG2a binding at titers as low as 1:480.
Figure 6. SP-D-TNFSFL adjuvants increase IgG2a antibody responses to Gag
Mice were immunized with HIV-1 Gag alone or combination of SP-D-TNFSFL plasmids, as indicated. Seven weeks after the third vaccination, mice were bled and serum from each group was serially diluted and assayed for IgG binding to p55 Gag antigen. Total IgG, IgG1, and IgG2a titers were measured by ELISA. (A) Total IgG response. (B) IgG2a response. (C) IgG1 response. SP-D-CD70, SP-D-OX40L, and SP-D-BAFF enhanced total IgG binding at 1:30 and 1:120 dilutions. SP-D-CD70 and SP-D-OX40L enhanced IgG2a binding at titers as low as 1:480.
Similar articles
- HIV-1 adenoviral vector vaccines expressing multi-trimeric BAFF and 4-1BBL enhance T cell mediated anti-viral immunity.
Kanagavelu S, Termini JM, Gupta S, Raffa FN, Fuller KA, Rivas Y, Philip S, Kornbluth RS, Stone GW. Kanagavelu S, et al. PLoS One. 2014 Feb 28;9(2):e90100. doi: 10.1371/journal.pone.0090100. eCollection 2014. PLoS One. 2014. PMID: 24587225 Free PMC article. - DNA vaccine molecular adjuvants SP-D-BAFF and SP-D-APRIL enhance anti-gp120 immune response and increase HIV-1 neutralizing antibody titers.
Gupta S, Clark ES, Termini JM, Boucher J, Kanagavelu S, LeBranche CC, Abraham S, Montefiori DC, Khan WN, Stone GW. Gupta S, et al. J Virol. 2015 Apr;89(8):4158-69. doi: 10.1128/JVI.02904-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631080 Free PMC article. - Improvement in T helper 1-related immune responses in BALB/c mice immunized with an HIV-1 gag plasmid combined with a chimeric plasmid encoding interleukin-18 and flagellin.
Chen YL, Chen YS, Hung YC, Liu PJ, Tasi HY, Ni WF, Hseuh PT, Lin HH. Chen YL, et al. Microbiol Immunol. 2015 Aug;59(8):483-94. doi: 10.1111/1348-0421.12274. Microbiol Immunol. 2015. PMID: 26094825 - Live virus vaccines based on a vesicular stomatitis virus (VSV) backbone: Standardized template with key considerations for a risk/benefit assessment.
Clarke DK, Hendry RM, Singh V, Rose JK, Seligman SJ, Klug B, Kochhar S, Mac LM, Carbery B, Chen RT; Brighton Collaboration Viral Vector Vaccines Safety Working Group. Clarke DK, et al. Vaccine. 2016 Dec 12;34(51):6597-6609. doi: 10.1016/j.vaccine.2016.06.071. Epub 2016 Jul 6. Vaccine. 2016. PMID: 27395563 Free PMC article. Review. - Old and new adjuvants.
McKee AS, Marrack P. McKee AS, et al. Curr Opin Immunol. 2017 Aug;47:44-51. doi: 10.1016/j.coi.2017.06.005. Epub 2017 Jul 19. Curr Opin Immunol. 2017. PMID: 28734174 Free PMC article. Review.
Cited by
- Design and selection of anti-PD-L1 single-domain antibody and tumor necrosis factor superfamily ligands for an optimal vectorization in an oncolytic virus.
Remy C, Pintado E, Dunlop M, Schön S, Kleinpeter P, Rozanes H, Fend L, Brandely R, Geist M, Suhner D, Winter E, Silvestre N, Huguet C, Fitzgerald P, Quéméneur E, Marchand JB. Remy C, et al. Front Bioeng Biotechnol. 2023 Nov 15;11:1247802. doi: 10.3389/fbioe.2023.1247802. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38053848 Free PMC article. - Agonism of 4-1BB for immune therapy: a perspective on possibilities and complications.
Salek-Ardakani S, Zajonc DM, Croft M. Salek-Ardakani S, et al. Front Immunol. 2023 Aug 17;14:1228486. doi: 10.3389/fimmu.2023.1228486. eCollection 2023. Front Immunol. 2023. PMID: 37662949 Free PMC article. Review. - Dietary Hesperidin Suppresses Lipopolysaccharide-Induced Inflammation in Male Mice.
Sato M, Okuno A, Ishisono K, Yajima Y, Toyoda A. Sato M, et al. Int J Tryptophan Res. 2022 Oct 28;15:11786469221128697. doi: 10.1177/11786469221128697. eCollection 2022. Int J Tryptophan Res. 2022. PMID: 36325028 Free PMC article. - Adenoviral delivery of soluble ovine OX40L or CD70 costimulatory molecules improves adaptive immune responses to a model antigen in sheep.
Rojas JM, Mancho C, Louloudes-Lázaro A, Rodríguez-Martín D, Avia M, Moreno S, Sevilla N, Martín V. Rojas JM, et al. Front Cell Infect Microbiol. 2022 Sep 23;12:1010873. doi: 10.3389/fcimb.2022.1010873. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36211974 Free PMC article. - Activation of OX40 and CD27 Costimulatory Signalling in Sheep through Recombinant Ovine Ligands.
Rojas JM, Alejo A, Avia JM, Rodríguez-Martín D, Sánchez C, Alcamí A, Sevilla N, Martín V. Rojas JM, et al. Vaccines (Basel). 2020 Jun 22;8(2):333. doi: 10.3390/vaccines8020333. Vaccines (Basel). 2020. PMID: 32580486 Free PMC article.
References
- Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, et al. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect Immun. 2006 Oct;74(10):5933–42. - PMC - PubMed
- Breathnach CC, Clark HJ, Clark RC, Olsen CW, Townsend HG, Lunn DP. Immunization with recombinant modified vaccinia Ankara (rMVA) constructs encoding the HA or NP gene protects ponies from equine influenza virus challenge. Vaccine. 2006 Feb 20;24(8):1180–90. - PubMed
- Li Z, Zhang M, Zhou C, Zhao X, Iijima N, Frankel FR. Novel vaccination protocol with two live mucosal vectors elicits strong cell-mediated immunity in the vagina and protects against vaginal virus challenge. J Immunol. 2008 Feb 15;180(4):2504–13. - PubMed
- Peters BS, Jaoko W, Vardas E, Panayotakopoulos G, Fast P, Schmidt C, et al. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine. 2007 Mar 1;25(11):2120–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1R21AI63982/AI/NIAID NIH HHS/United States
- R21AI088511/AI/NIAID NIH HHS/United States
- R21 AI088511/AI/NIAID NIH HHS/United States
- 1R21AI073240/AI/NIAID NIH HHS/United States
- K22 AI068489-02/AI/NIAID NIH HHS/United States
- P30 AI073961/AI/NIAID NIH HHS/United States
- K22 AI068489-01A2/AI/NIAID NIH HHS/United States
- 1K22AI068489/AI/NIAID NIH HHS/United States
- 1P30AI073961/AI/NIAID NIH HHS/United States
- R21 AI063982/AI/NIAID NIH HHS/United States
- R21 AI078834-01/AI/NIAID NIH HHS/United States
- 1R21AI078834/AI/NIAID NIH HHS/United States
- R21 AI078834-02/AI/NIAID NIH HHS/United States
- R21 AI073240/AI/NIAID NIH HHS/United States
- K22 AI068489/AI/NIAID NIH HHS/United States
- R21 AI078834/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials