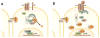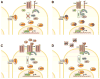Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers - PubMed (original) (raw)
Review
Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers
Bryan D White et al. Gastroenterology. 2012 Feb.
Abstract
Aberrant Wnt/β-catenin signaling is widely implicated in numerous malignancies, including cancers of the gastrointestinal tract. Dysregulation of signaling is traditionally attributed to mutations in Axin, adenomatous polyposis coli, and β-catenin that lead to constitutive hyperactivation of the pathway. However, Wnt/β-catenin signaling is also modulated through various other mechanisms in cancer, including cross talk with other altered signaling pathways. A more complex view of Wnt/β-catenin signaling and its role in gastrointestinal cancers is now emerging as divergent phenotypic outcomes are found to be dictated by temporospatial context and relative levels of pathway activation. This review summarizes the dysregulation of Wnt/β-catenin signaling in colorectal carcinoma, hepatocellular carcinoma, and pancreatic ductal adenocarcinoma, with particular emphasis on the latter two. We conclude by addressing some of the major challenges faced in attempting to target the pathway in the clinic.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
No conflicts of interest exist.
Figures
Figure 1
Schematic illustrating the Wnt/β-catenin pathway. (A) In the absence of a Wnt signal, β-catenin is bound to E-Cadherin (E-CAD) at adherens junctions or is phosphorylated by a destruction complex comprised of the core proteins AXIN, APC (adenomatous polyposis coli), GSK3 (glycogen synthase kinase), and CK1 (casein kinase 1). N-terminal phosphorylated β-catenin is targeted for ubiquitination and subsequent proteasomal degradation, maintaining low levels of cystosolic and nuclear β-catenin. Expression of Wnt/β-catenin target genes via activation of TCF/LEF (T-cell factor/lymphoid enhancer factor) transcription factors is inhibited by the transcriptional repressor Groucho. (B) Wnt ligand initiates signaling through FZD (Frizzled) receptor and LRP (low-density lipoprotein receptor-related protein) co-receptor, activating and recruiting DVL (Dishevelled) and Axin to the membrane, thereby disrupting the destruction complex. Higher cytosolic levels of β-catenin result in its translocation into the nucleus, where it binds TCF/LEF transcription factors and displaces Groucho to trans-activate Wnt/β-catenin target gene expression.
Figure 2
Common mechanisms by which the Wnt/β-catenin pathway is dysregulated in cancer. (A) Loss-of-function mutations in APC (adenomatous polyposis coli) lead to a breakdown of the destruction complex, accumulation of β-catenin in the cytoplasm, translocation of β-catenin into the nucleus, and constitutive expression of Wnt/β-catenin-dependent genes. (B) Gain-of-function mutations in β-catenin, often occurring in exon 3, prevent its N-terminal phosphorylation thus averting its ubiquitination and degradation. (C) Overexpression of FZD (frizzled) receptors or WNT ligands can lead to increased activation of the pathway. (D) Underexpression of secreted inhibitors of the pathway (i.e., secreted frizzled-related proteins, sFRPs) can also lead to increased sensitivity to Wnt ligands and increased pathway activation.
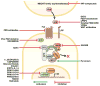
Figure 3
Additional mechanisms by which Wnt/β-catenin signaling can be modulated in cancer. (A) In hepatocytes, β-catenin can be released from an additional membrane bound pool associated with the receptor C-MET. When engaged by HGF (hepatocyte growth factor), C-MET releases β-catenin into the cytoplasm with its eventual translocation into the nucleus. (B) Membrane-bound NOTCH1 can bind activated β-catenin and cause its lysosomal destruction, thereby inhibiting Wnt/β-catenin signaling. A decrease in NOTCH1 expression may therefore potentiate Wnt/β-catenin signaling in certain types of cancer. (C) Interactions with numerous proteins can modulate Wnt/β-catenin signaling at various levels in the pathway. SULF-1 (Sulfatase-1) can increase the efficiency of Wnt ligands by modulating their interactions with heparin sulfate proteoglycans in the extracellular environment. ATDC (Ataxia telangiectasia group D-complementing) protein can potentiate pathway activation through its interaction with DVL (Dishevelled). SMO (Smoothened) can increase Wnt/β-catenin signaling by an unknown mechanism. Undoubtedly, novel mechanisms will be uncovered to explain the complex context dependency of Wnt/β-catenin signaling.
Figure 4
Schematic illustrating numerous small molecules and biological compounds identified in the literature that inhibit Wnt/β-catenin signaling at various points in the pathway.
Similar articles
- Tumor suppressor Fbxw7 antagonizes WNT signaling by targeting β-catenin for degradation in pancreatic cancer.
Jiang JX, Sun CY, Tian S, Yu C, Chen MY, Zhang H. Jiang JX, et al. Tumour Biol. 2016 Oct;37(10):13893-13902. doi: 10.1007/s13277-016-5217-5. Epub 2016 Aug 3. Tumour Biol. 2016. PMID: 27485116 - CUL4B promotes metastasis and proliferation in pancreatic cancer cells by inducing epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway.
He YM, Xiao YS, Wei L, Zhang JQ, Peng CH. He YM, et al. J Cell Biochem. 2018 Jul;119(7):5308-5323. doi: 10.1002/jcb.26643. Epub 2018 Mar 14. J Cell Biochem. 2018. PMID: 29274277 Retracted. - MicroRNA-1229 overexpression promotes cell proliferation and tumorigenicity and activates Wnt/β-catenin signaling in breast cancer.
Tan Z, Zheng H, Liu X, Zhang W, Zhu J, Wu G, Cao L, Song J, Wu S, Song L, Li J. Tan Z, et al. Oncotarget. 2016 Apr 26;7(17):24076-87. doi: 10.18632/oncotarget.8119. Oncotarget. 2016. PMID: 26992223 Free PMC article. - Mucins and Wnt/β-catenin signaling in gastrointestinal cancers: an unholy nexus.
Pai P, Rachagani S, Dhawan P, Batra SK. Pai P, et al. Carcinogenesis. 2016 Mar;37(3):223-32. doi: 10.1093/carcin/bgw005. Epub 2016 Jan 13. Carcinogenesis. 2016. PMID: 26762229 Free PMC article. Review. - Wnt signaling in adult intestinal stem cells and cancer.
Krausova M, Korinek V. Krausova M, et al. Cell Signal. 2014 Mar;26(3):570-9. doi: 10.1016/j.cellsig.2013.11.032. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308963 Review.
Cited by
- Differential Effects of Trp53 Alterations in Murine Colorectal Cancer.
Betzler AM, Nanduri LK, Hissa B, Blickensdörfer L, Muders MH, Roy J, Jesinghaus M, Steiger K, Weichert W, Kloor M, Klink B, Schroeder M, Mazzone M, Weitz J, Reissfelder C, Rahbari NN, Schölch S. Betzler AM, et al. Cancers (Basel). 2021 Feb 15;13(4):808. doi: 10.3390/cancers13040808. Cancers (Basel). 2021. PMID: 33671932 Free PMC article. - Aberrant regulation of Wnt signaling in hepatocellular carcinoma.
Liu LJ, Xie SX, Chen YT, Xue JL, Zhang CJ, Zhu F. Liu LJ, et al. World J Gastroenterol. 2016 Sep 7;22(33):7486-99. doi: 10.3748/wjg.v22.i33.7486. World J Gastroenterol. 2016. PMID: 27672271 Free PMC article. Review. - Cables1 is a tumor suppressor gene that regulates intestinal tumor progression in Apc(Min) mice.
Arnason T, Pino MS, Yilmaz O, Kirley SD, Rueda BR, Chung DC, Zukerberg LR. Arnason T, et al. Cancer Biol Ther. 2013 Jul;14(7):672-8. doi: 10.4161/cbt.25089. Epub 2013 May 31. Cancer Biol Ther. 2013. PMID: 23792637 Free PMC article. - Hsp70 exerts oncogenic activity in the Apc mutant Min mouse model.
Tao Y, Messer JS, Goss KH, Hart J, Bissonnette M, Chang EB. Tao Y, et al. Carcinogenesis. 2016 Jul;37(7):731-739. doi: 10.1093/carcin/bgw056. Epub 2016 May 4. Carcinogenesis. 2016. PMID: 27207671 Free PMC article. - PYK2 Is Involved in Premalignant Acinar Cell Reprogramming and Pancreatic Ductal Adenocarcinoma Maintenance by Phosphorylating β-CateninY654.
Gao C, Chen G, Zhang DH, Zhang J, Kuan SF, Hu W, Esni F, Gao X, Guan JL, Chu E, Hu J. Gao C, et al. Cell Mol Gastroenterol Hepatol. 2019;8(4):561-578. doi: 10.1016/j.jcmgh.2019.07.004. Epub 2019 Jul 19. Cell Mol Gastroenterol Hepatol. 2019. PMID: 31330317 Free PMC article.
References
- Moon RT, Kohn AD, De Ferrari GV, et al. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. - PubMed
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources