Molecular programming of B cell memory - PubMed (original) (raw)
Review
Molecular programming of B cell memory
Michael McHeyzer-Williams et al. Nat Rev Immunol. 2011.
Abstract
The development of high-affinity B cell memory is regulated through three separable phases, each involving antigen recognition by specific B cells and cognate T helper cells. Initially, antigen-primed B cells require cognate T cell help to gain entry into the germinal centre pathway to memory. Once in the germinal centre, B cells with variant B cell receptors must access antigens and present them to germinal centre T helper cells to enter long-lived memory B cell compartments. Following antigen recall, memory B cells require T cell help to proliferate and differentiate into plasma cells. A recent surge of information - resulting from dynamic B cell imaging in vivo and the elucidation of T follicular helper cell programmes - has reshaped the conceptual landscape surrounding the generation of memory B cells. In this Review, we integrate this new information about each phase of antigen-specific B cell development to describe the newly unravelled molecular dynamics of memory B cell programming.
Figures
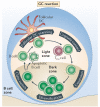
Figure 1. TFH cell-regulated memory B cell development
a | Local protein vaccination induces dendritic cell (DC) maturation and migration to the T cell zones of draining lymph nodes. DCs that express peptide–MHC class II complexes engage naive, antigen-specific CD4+ T cells to induce their proliferation and differentiation into effector T helper (TH) cells. In the B cell zone, whole antigen is trapped by subcapsular sinus (SCS) macrophages and presented to naive follicular B cells. Antigen-specific B cells become activated, take up, process and present antigenic peptides and migrate towards the B cell–T cell borders of the draining lymph node. Effector TH cells emerge in multiple forms; emigrant TH cells exit the lymph node to function at distal tissue sites and T follicular helper (TFH) cells relocate to B cell–T cell borders and interfollicular regions. Cognate contact between pre-germinal centre (pre-GC) TFH cells and antigen-primed B cells is required for multiple programming events in the pathway to B cell memory. b | Clonal expansion, antibody class switching and non-GC plasma cell development proceeds in the extrafollicular regions of the lymph nodes. Secondary follicle formation and antibody class switching precede the initiation of the GC reaction, which forms the dominant pathway for the generation of memory B cells. c | Polarization of the secondary follicle anatomically signifies the initiation of the GC cycle. The dark zone supports GC centroblast proliferation, class-switch recombination and B cell receptor (BCR) diversification through somatic hypermutation. Non-cycling GC centrocytes move to the light zone and continually scan follicular DC networks. Centrocytes that lose the ability to bind to the presented antigen undergo apoptosis, while those that express a variant BCR with a higher affinity can compete for binding to antigen-specific GC TFH cells. Cognate contact with GC TFH cells requires peptide–MHC class II expression by the GC centrocytes. This contact can promote B cell re-entry into the dark zone and the GC cycle or exit from the GC and entry into the affinity-matured memory B cell compartments of non-secreting memory B cells and post-GC plasma cells. d | Following antigen re-challenge, memory B cells present antigens to memory TFH cells to promote memory B cell clonal expansion with rapid memory plasma cell generation and the induction of a secondary GC reaction. Although related to the cellular and molecular activities of the primary response, as depicted, the memory-response dynamics remain poorly resolved. TCR, T cell receptor.
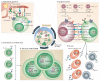
Figure 2. Pre-GC phase: commitment to memory
a | Multiple subsets of antigen-specific pre-germinal centre (pre-GC) T follicular helper (TFH) cells are produced to regulate B cell immunity. So far, the organization of these subsets remains speculative; there is evidence for distinct TFH cell populations that secrete different cytokines and regulate commitment to separate antibody classes, as well as for other types of TFH cells that regulate non-GC plasma cell differentiation. Expression of B cell lymphoma 6 (BCL-6) and CXC-chemokine receptor 5 (CXCR5) is thought to be a common feature of all TFH cell subsets. b | Antigen-primed B cells must process and present peptide–MHC class II complexes to receive cognate help from pre-GC TFH cells. Upregulation of the molecules involved in TFH cell contact is a poorly resolved component of early antigen-driven B cell maturation. Cognate contact between antigen-specific T cell receptors (TCRs) and peptide–MHC class II complexes focuses the intercellular exchange of molecular information between pre-GC B cells and TFH cells. The modifying interactions that occur at first contact are known to involve co-stimulatory molecule interactions (for example, CD40L–CD40 and inducible T cell co-stimulator (ICOS)–ICOSL), accessory molecule interactions (for example, SLAM family interactions and OX40–OX40L) and interactions between cytokines and their receptors (for example, interleukin-4 (IL-4)–IL-4R, interferon-γ (IFNγ)–IFNγR and IL-21–IL-21R). The distribution of these functional attributes in pre-GC TFH cell compartments is not yet well resolved in vivo. c | The non-GC pathway to plasma cell development permits antibody class-switch recombination without somatic hypermutation, and the outcome depends largely on the cytokine stimulus provided by pre-GC TFH cells. B lymphocyte-induced maturation protein 1 (BLIMP1) expression is required for plasma cell commitment across all antibody classes. The GC pathway to memory B cell development begins with extensive B cell proliferation in secondary follicles that polarize into dark and light zones to initiate the GC reaction. The GC pathway is associated with BCL-6 upregulation and AID expression to support both class-switch recombination and somatic hypermutation. These GC features enable the generation of all antibody classes and require a long duration of productive contact with pre-GC TFH cells. TH, T helper.
Figure 3. The antigen-specific GC reaction
The germinal centre (GC) cycle is initiated through the pre-GC contact of B cells with cognate T follicular helper (TFH) cells, as this promotes the extensive proliferation of antigen-primed B cells. The GC cycle is thought to begin when an IgD− secondary follicle polarizes to form two microanatomically distinct regions: the T cell zone-proximal dark zone (which contains proliferating centroblasts) and the T cell zone-distal light zone (which contains centrocytes, antigen-laden follicular dendritic cell (DC) networks and antigen-specific GC TFH cells). The clonal expansion of antigen-specific GC B cells in the dark zone is accompanied by B cell receptor (BCR) diversification through somatic hypermutation, which introduces point mutations into the variable-region segments of antibody genes. Antibody class-switch recombination can also proceed under these circumstances. Both somatic hypermutation and class-switch recombination are associated with transcriptionally active gene loci, require DNA replication and repair machinery and occur during the cell cycle. Hence, these activities have been associated with the dark-zone phase of the GC cycle. Exit from the cell cycle coincides with the relocation of non-cycling GC B cells to the light zone. Continual scanning of follicular DCs that are coated with immune complexes is observed in the light zone and has been associated with the potential for GC B cells to test their variant BCRs for antigen-binding ability. Loss of antigen binding can lead to death by apoptosis and the clearance of dead cells by tingible body macrophages in the light zone. Positive signals through the BCR during the scanning of follicular DCs program GC B cells to compete for contact with cognate GC TFH cells. Productive contact with GC TFH cells can induce re-entry into the GC cycle; this involves movement back into the dark zone, the induction of the cell cycle and BCR re-diversification. Alternatively, affinity-matured GC B cells can exit the GC, either as non-secreting memory B cell precursors for the memory response, or as secreting long-lived memory plasma cells that contribute to serological memory.
Figure 4. Memory B cell evolution
a | Cues from pre-germinal centre (pre-GC) cognate T follicular helper (TFH) cells instruct antigen-primed B cells to initiate the GC reaction. It is likely that the commitment to antibody class is pre-programmed at this initial juncture and that all classes of B cells can seed the primary GC response. b | Molecular control of the cell cycle is an integral component of dark-zone B cell dynamics and involves the expression of B cell lymphoma 6 (BCL-6), although the ways in which BCL-6 contributes to this regulation remain poorly resolved. The expression and activity of activation-induced cytidine deaminase (AID) and uracil DNA glycosylase (UNG) are required to initiate somatic hypermutation (SHM), which is targeted to single-stranded DNA. Following uracil excision, the DNA is processed by error-prone DNA polymerases to introduce point mutations into the variable regions of the rearranged antibody genes. Class-switch recombination (CSR) can also occur during this dark-zone phase using AID to target DNA cleavage to antibody switch regions; the DNA double strand breaks that are generated trigger the DNA damage machinery, which completes the CSR event. The associations between cell cycle control, SHM and CSR are not clearly resolved in vivo. c | To scan folicullar dendritic cells (DCs) for antigens, GC B cells continuously move along follicular DC processes that are laden with mature immune complexes. These interactions are more similar to stromal cell-associated trafficking behaviour than to stable immune synapse-like interactions. The affinity of the B cell receptor (BCR) for antigens may influence antigen uptake and peptide–MHC class II presentation at this juncture of development. Programmes of gene expression for molecules that are able to modify cognate contact may also be differentially induced as a result of BCR signal strength during follicular DC scanning. d | B cells then make contact for a longer duration with cognate GC TFH cells in the light zone, and this can be visualized directly in vivo. As in earlier, pre-GC events, these contacts must focus around T cell receptor (TCR)–peptide–MHC class II interactions and can be modified by a multitude of intercellular exchanges of molecular information. There is still little detailed analysis of these interactions in vivo. We depict the classes of molecules that can be associated with this crucial programming event, but the organization of these interactions and their precise developmental imprint are not yet clear. e | Antigen presentation by B cells can influence re-entry into the dark zone and the re-initiation of BCR diversification (which involves cell proliferation, SHM and CSR). f | GC cognate contact can also initiate B cell exit from the GC into the distinct non-secreting memory B cell and post-GC long-lived memory plasma cell compartments. BLIMP1, B lymphocyte-induced maturation protein 1; CR2, complement receptor 2; CXCR5, CXC-chemokine receptor 5; IFNγ, interferon-γ; IL, interleukin; PD1, programmed cell death protein 1; TH, T helper.
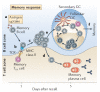
Figure 5. Memory response to antigen recall
a | Memory B cell responses can emerge in the absence of innate inflammatory stimuli. In this case, the main antigen-presenting cells are the affinity-matured memory B cells themselves. b | The memory B cell response to T cell-dependent antigens still requires T helper (TH) cell-mediated regulation following antigen recall. When the priming and recall antigens are identical, memory TH cells are the rapid responders and are thought to emerge preferentially over their low-frequency naive counterparts. Regarding the regulation of memory B cell responses, antigen-specific memory T follicular helper (TFH) cells are the most likely candidates for rapid cognate regulation. c | Cognate contact at this developmental juncture occurs across sets of memory B cells and memory TFH cells, but the organization and kinetics of this process remain poorly resolved in vivo. There is rapid and vigorous local clonal expansion during the first 2–3 days after antigen exposure in both the memory B cell and memory TFH cell compartments. d | Proliferation of affinity-matured memory plasma cells occurs very quickly, and evidence suggests that most memory plasma cells have already undergone affinity maturation. e | There is evidence for memory B cell subsets that have a germinal centre (GC) phenotype and create GC-like structures following antigen recall. Whether these structures are residual from the primary-response GC or re-emerge with GC activities following recall has not been resolved. f | Increased numbers of memory B cells and memory-response plasma cells persist after antigen recall. It remains unclear whether these cells are the product of memory GC reactions or of the extrafollicular, non-GC memory response. DC, dendritic cell; TCR, T cell receptor.
Similar articles
- Follicular helper T cells as cognate regulators of B cell immunity.
McHeyzer-Williams LJ, Pelletier N, Mark L, Fazilleau N, McHeyzer-Williams MG. McHeyzer-Williams LJ, et al. Curr Opin Immunol. 2009 Jun;21(3):266-73. doi: 10.1016/j.coi.2009.05.010. Epub 2009 Jun 6. Curr Opin Immunol. 2009. PMID: 19502021 Free PMC article. Review. - Here, there and everywhere: T follicular helper cells on the move.
Ma CS, Phan TG. Ma CS, et al. Immunology. 2017 Nov;152(3):382-387. doi: 10.1111/imm.12793. Epub 2017 Aug 7. Immunology. 2017. PMID: 28704588 Free PMC article. Review. - Antigen-specific memory B cell development.
McHeyzer-Williams LJ, McHeyzer-Williams MG. McHeyzer-Williams LJ, et al. Annu Rev Immunol. 2005;23:487-513. doi: 10.1146/annurev.immunol.23.021704.115732. Annu Rev Immunol. 2005. PMID: 15771579 Review. - TFH cells in bystander and cognate interactions with B cells.
Wan Z, Lin Y, Zhao Y, Qi H. Wan Z, et al. Immunol Rev. 2019 Mar;288(1):28-36. doi: 10.1111/imr.12747. Immunol Rev. 2019. PMID: 30874359 Review. - T follicular helper cells in space-time.
Qi H. Qi H. Nat Rev Immunol. 2016 Oct;16(10):612-25. doi: 10.1038/nri.2016.94. Epub 2016 Aug 30. Nat Rev Immunol. 2016. PMID: 27573485 Review.
Cited by
- Modulation of Primary Immune Response by Different Vaccine Adjuvants.
Ciabattini A, Pettini E, Fiorino F, Pastore G, Andersen P, Pozzi G, Medaglini D. Ciabattini A, et al. Front Immunol. 2016 Oct 17;7:427. doi: 10.3389/fimmu.2016.00427. eCollection 2016. Front Immunol. 2016. PMID: 27781036 Free PMC article. - Multivalent Antigens for Promoting B and T Cell Activation.
Bennett NR, Zwick DB, Courtney AH, Kiessling LL. Bennett NR, et al. ACS Chem Biol. 2015 Aug 21;10(8):1817-24. doi: 10.1021/acschembio.5b00239. Epub 2015 Jun 2. ACS Chem Biol. 2015. PMID: 25970017 Free PMC article. - Expansion of HIV-specific T follicular helper cells in chronic HIV infection.
Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, Davis I, Farber D, Hartjen P, Haag F, Alter G, Schulze zur Wiesch J, Streeck H. Lindqvist M, et al. J Clin Invest. 2012 Sep;122(9):3271-80. doi: 10.1172/JCI64314. Epub 2012 Aug 27. J Clin Invest. 2012. PMID: 22922259 Free PMC article. - How the immune system talks to itself: the varied role of synapses.
Xie J, Tato CM, Davis MM. Xie J, et al. Immunol Rev. 2013 Jan;251(1):65-79. doi: 10.1111/imr.12017. Immunol Rev. 2013. PMID: 23278741 Free PMC article. Review. - Prospective study and validation of early warning marker discovery based on integrating multi-omics analysis in severe burn patients with sepsis.
Huang J, Chen Y, Guo Z, Yu Y, Zhang Y, Li P, Shi L, Lv G, Sun B. Huang J, et al. Burns Trauma. 2023 Jan 15;11:tkac050. doi: 10.1093/burnst/tkac050. eCollection 2023. Burns Trauma. 2023. PMID: 36659877 Free PMC article.
References
- Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. - PubMed
- Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. - PubMed
- Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nature Rev. Immunol. 2009;9:15–27. - PubMed
- Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases