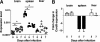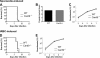Experimental cerebral malaria develops independently of caspase recruitment domain-containing protein 9 signaling - PubMed (original) (raw)
Experimental cerebral malaria develops independently of caspase recruitment domain-containing protein 9 signaling
Julius Clemence R Hafalla et al. Infect Immun. 2012 Mar.
Abstract
The outcome of infection depends on multiple layers of immune regulation, with innate immunity playing a decisive role in shaping protection or pathogenic sequelae of acquired immunity. The contribution of pattern recognition receptors and adaptor molecules in immunity to malaria remains poorly understood. Here, we interrogate the role of the caspase recruitment domain-containing protein 9 (CARD9) signaling pathway in the development of experimental cerebral malaria (ECM) using the murine Plasmodium berghei ANKA infection model. CARD9 expression was upregulated in the brains of infected wild-type (WT) mice, suggesting a potential role for this pathway in ECM pathogenesis. However, P. berghei ANKA-infected Card9(-/-) mice succumbed to neurological signs and presented with disrupted blood-brain barriers similar to WT mice. Furthermore, consistent with the immunological features associated with ECM in WT mice, Card9(-/-) mice revealed (i) elevated levels of proinflammatory responses, (ii) high frequencies of activated T cells, and (iii) CD8(+) T cell arrest in the cerebral microvasculature. We conclude that ECM develops independently of the CARD9 signaling pathway.
Figures
Fig 1
CARD9 expression during a Plasmodium berghei (strain ANKA) infection. RT-PCR of CARD9 expression was performed using total RNA isolated from brains, spleens, and livers of uninfected mice and on days 5 and 7 from P. berghei ANKA-infected mice and using primers specific for the CARD9 coding sequence. Results were normalized to amplification products using GAPDH primers. (A) Relative expression levels from two to three experiments. *, P < 0.05 following Kruskal-Wallis test and Dunn's multiple comparison test. (B) Mean fold change shown as cumulative data from two experiments.
Fig 2
_Card9_−/− mice develop ECM similar to WT mice. (A) ECM development after sporozoite-induced infection by intravenous injection of 104P. berghei ANKA sporozoites. Survival curves are based on cumulative data from two independent experiments, with five mice per group. (B) Time to blood stage infection (prepatency) after intravenous injection of 104P. berghei ANKA sporozoites. (C) Parasitemia levels (for panel A) determined by Giemsa-stained blood smears. (D) ECM development after transfusion-mediated infection by intravenous injection of 104P. berghei ANKA-infected red blood cells (iRBCs). Survival curves are based on cumulative data from three independent experiments, with four to six mice per group. (E) Parasitemia levels (for panel D) determined by Giemsa-stained blood smears.
Fig 3
_Card9_−/− mice display systemic cytokine responses similar to those of WT mice. Cytokine concentrations in plasma samples from infected mice. Cytokines were determined using the BD cytometric bead assay inflammation kit. Data shown are for IL-17 (A), IL-2 (B), IL-4 (C), IL-6 (D), IL-10 (E), IFN-γ (F), MCP-1 (G), and TNF-α (H). Day 0 represent data from uninfected mice. Figures show representative data from two to three experiments, four mice per group.
Fig 4
Systemic cellular responses and leukocyte sequestration to the brain in WT and _Card9_−/− mice infected with P. berghei ANKA. (A to D) High proportion of antigen-experienced CD8+ and CD4+ T cells in spleens after infection. Splenic leukocytes were isolated from uninfected mice and infected WT and _Card9_−/− mice on day 7 and surface stained for CD11a (A, B) or CD62L (C, D) and CD8+ or CD4+. (A, C) Shown are representative fluorescence-activated cell sorting (FACS) plots displaying the proportions of CD11ahi (A) or CD62Llo (C) CD8+ (left) and CD4+(right) T cells before and 7 days after infection with 104P. berghei ANKA-infected iRBCs. (B, D) Graphs show the percentage (mean ± standard error of the mean [SEM]) of CD11ahi or CD62Llo cells of total splenic CD8+ (left) and CD4+ (right) T cells before and 7 days after infection. Data are from one representative out of two experiments, with at least four mice per group. (E, F) High proportion of IFN-γ-producing cells in spleens after infection. Following splenic leukocyte isolation, cells were cultured with anti-CD3 and anti-CD28 antibodies for 5 h in the presence of brefeldin A. Cells were subsequently stained for surface CD8+ or CD4+ and intracellular IFN-γ. (E) Shown are representative FACS plots displaying the proportions of IFN-γ+ CD8+ (left) and CD4+(right) T cells before and 7 days after infection with 104P. berghei ANKA-infected iRBCs. (F) Graphs show the percentage (mean ± SEM) of IFN-γ+ cells of total splenic CD8+ (left) and CD4+ (right) T cells before and 7 days after infection. Data are from one representative out of two experiments, with at least four mice per group. (G, H) High proportion of infiltrating leukocytes in brains after infection. Brain-sequestered lymphocytes were isolated from uninfected and day 7 infected WT and _Card9_−/− mice. Cells were surface stained for CD8 and CD4. (G) Representative FACS plots show the proportions of CD8+ leukocytes before and 7 days after infection with 104P. berghei ANKA-infected iRBCs. (H) Graphs show the percentage (mean ± SEM) of CD8+ leukocytes before and 7 days after infection. Data are from one representative out of three experiments, with at least four mice per group.
Similar articles
- Disruption of Parasite hmgb2 Gene Attenuates Plasmodium berghei ANKA Pathogenicity.
Briquet S, Lawson-Hogban N, Boisson B, Soares MP, Péronet R, Smith L, Ménard R, Huerre M, Mécheri S, Vaquero C. Briquet S, et al. Infect Immun. 2015 Jul;83(7):2771-84. doi: 10.1128/IAI.03129-14. Epub 2015 Apr 27. Infect Immun. 2015. PMID: 25916985 Free PMC article. - Critical role of IL-33 receptor ST2 in experimental cerebral malaria development.
Palomo J, Reverchon F, Piotet J, Besnard AG, Couturier-Maillard A, Maillet I, Tefit M, Erard F, Mazier D, Ryffel B, Quesniaux VF. Palomo J, et al. Eur J Immunol. 2015 May;45(5):1354-65. doi: 10.1002/eji.201445206. Epub 2015 Mar 20. Eur J Immunol. 2015. PMID: 25682948 - Establishment of a murine model of cerebral malaria in KunMing mice infected with Plasmodium berghei ANKA.
Ding Y, Xu W, Zhou T, Liu T, Zheng H, Fu Y. Ding Y, et al. Parasitology. 2016 Oct;143(12):1672-80. doi: 10.1017/S0031182016001475. Epub 2016 Aug 30. Parasitology. 2016. PMID: 27574013 - Genetic analysis of cerebral malaria in the mouse model infected with Plasmodium berghei.
Torre S, Langlais D, Gros P. Torre S, et al. Mamm Genome. 2018 Aug;29(7-8):488-506. doi: 10.1007/s00335-018-9752-9. Epub 2018 Jun 19. Mamm Genome. 2018. PMID: 29922917 Review. - Unravelling the roles of innate lymphoid cells in cerebral malaria pathogenesis.
Palomo J, Quesniaux VFJ, Togbe D, Reverchon F, Ryffel B. Palomo J, et al. Parasite Immunol. 2018 Feb;40(2). doi: 10.1111/pim.12502. Parasite Immunol. 2018. PMID: 29117626 Review.
Cited by
- Interferons and interferon regulatory factors in malaria.
Gun SY, Claser C, Tan KS, Rénia L. Gun SY, et al. Mediators Inflamm. 2014;2014:243713. doi: 10.1155/2014/243713. Epub 2014 Jul 15. Mediators Inflamm. 2014. PMID: 25157202 Free PMC article. Review. - Signalling C-type lectin receptors, microbial recognition and immunity.
Hoving JC, Wilson GJ, Brown GD. Hoving JC, et al. Cell Microbiol. 2014 Feb;16(2):185-94. doi: 10.1111/cmi.12249. Epub 2014 Jan 10. Cell Microbiol. 2014. PMID: 24330199 Free PMC article. Review. - Plasmodium berghei sporozoites acquire virulence and immunogenicity during mosquito hemocoel transit.
Sato Y, Montagna GN, Matuschewski K. Sato Y, et al. Infect Immun. 2014 Mar;82(3):1164-72. doi: 10.1128/IAI.00758-13. Epub 2013 Dec 30. Infect Immun. 2014. PMID: 24379288 Free PMC article. - Involvement of Nod2 in the innate immune response elicited by malarial pigment hemozoin.
Corbett Y, Parapini S, D'Alessandro S, Scaccabarozzi D, Rocha BC, Egan TJ, Omar A, Galastri L, Fitzgerald KA, Golenbock DT, Taramelli D, Basilico N. Corbett Y, et al. Microbes Infect. 2015 Mar;17(3):184-94. doi: 10.1016/j.micinf.2014.11.001. Epub 2014 Nov 21. Microbes Infect. 2015. PMID: 25462568 Free PMC article. - CARD9 in host immunity to fungal, bacterial, viral, and parasitic infections: An update.
Hu A, Hu Z, Zou H, Zhang J, Zhang D, Wang H, Zhong J, Chen B. Hu A, et al. Front Microbiol. 2022 Nov 9;13:1021837. doi: 10.3389/fmicb.2022.1021837. eCollection 2022. Front Microbiol. 2022. PMID: 36439825 Free PMC article. Review.
References
- Amani V, et al. 2000. Involvement of IFN-gamma receptor-mediated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur. J. Immunol. 30:1646–1655 - PubMed
- Belnoue E, et al. 2002. On the pathogenic role of brain-sequestered alpha beta CD8+ T cells in experimental cerebral malaria. J. Immunol. 169:6369–6375 - PubMed
- Bertin J, et al. 2000. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-kappa B. J. Biol. Chem. 275:41082–41086 - PubMed
- Coban C, et al. 2007. Pathological role of Toll-like receptor signaling in cerebral malaria. Int. Immunol. 19:67–79 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials