Empathy and pro-social behavior in rats - PubMed (original) (raw)
Empathy and pro-social behavior in rats
Inbal Ben-Ami Bartal et al. Science. 2011.
Erratum in
- Science. 2012 Jan 27;335(6067):401
Abstract
Whereas human pro-social behavior is often driven by empathic concern for another, it is unclear whether nonprimate mammals experience a similar motivational state. To test for empathically motivated pro-social behavior in rodents, we placed a free rat in an arena with a cagemate trapped in a restrainer. After several sessions, the free rat learned to intentionally and quickly open the restrainer and free the cagemate. Rats did not open empty or object-containing restrainers. They freed cagemates even when social contact was prevented. When liberating a cagemate was pitted against chocolate contained within a second restrainer, rats opened both restrainers and typically shared the chocolate. Thus, rats behave pro-socially in response to a conspecific's distress, providing strong evidence for biological roots of empathically motivated helping behavior.
Figures
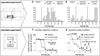
Fig. 1
Rats showed activity focused on the restrainer (red box in A) only if it contained a trapped cagemate. Rats were placed in an arena with a trapped cagemate (n=30, 6 females), an empty restrainer (n = 20, 6 females) a restrainer containing an object (n=8 males) or with an empty restrainer and a free cagemate located across a perforated screen (n=12 males). (A) The locations, plotted at 0.5 Hz, of a representative free rat from each of the 4 groups on the first day of testing are illustrated. (B) The mean (±SEM) amount of time that a rat was >5 cm away from the arena wall is shown for each of the 12 days of testing. Rats in the trapped cagemate condition spent more time in the arena center than did rats in the control conditions (P<0.001, mixed model analysis, post-hoc PLSD). (C) The mean (±SEM) activity across the testing session (averaged across all days) for each group of rats is shown. Rats in the trapped cagemate condition moved significantly faster than rats in all other conditions (P<0.001, mixed model analysis, post-hoc PLSD). (D) The mean ratio (±SEM) of the average activity during the second half of sessions relative to the average activity during the first half is shown for each day of testing. On days 1–6, rats in the trapped cagemate condition stayed significantly more active in the second half relative to the first half of sessions than did rats in the control conditions (P<0.001 mixed model analysis, PLSD).
Fig. 2
Rats placed with a trapped cagemate learned to open the restrainer door in a goal-directed fashion. (A) The proportion of rats in the trapped cagemate condition that opened the door increased across the days of testing. (B) The median time to door-opening is shown across all days of testing. Only rats in the trapped cagemate condition opened the door at decreasing latencies. The dashed line indicates the time point when the door was opened half way by the experimenter. (C) Rats in the trapped cagemate condition showed a sharp increase in activity at the moment that the restrainer door was opened (time 0). Activity from all trials, regardless of whether the door was opened before or after the experimenter opened the door halfway, was averaged. (D) The count of opening type (with head, from side, from top) is shown across days of testing. As free rats learned to open the door, they developed a consistent style, suggesting goal-directed behavior. (E) Initially, rats showed freezing behavior immediately after the door fell over, but as they learned to open the door, they were no longer startled by the door-opening and no longer froze. (F) More alarm calls were recorded in the trapped cagemate condition (n=67 sample files) than in the empty (n=64) and object (n= 67) conditions (p<0.05 ANOVA, PLSD<0.05).
Fig. 3
(A) Females in the trapped cagemate condition (n=6) opened the door at consistently shorter latencies than did males (n=24) on days 7–12 (P<0.01, Mixed model analysis). When median door-opening latencies on days 7–12 were averaged, there was no difference between males (n=14) and females (n=6) in the empty condition. (B) The mean (±SEM) activity was greater for females than males in the trapped cagemate condition (P<0.001, ANOVA) but there was no gender-related difference in the empty condition.
Fig. 4
(A) In the separated experiment, rats (n=9) opened the door for a trapped cagemate even when no contact was possible between the two animals after door-opening. The latencies to door-opening are plotted across all the days of the study. Rats extinguished door-opening when the restrainer was empty, but opened the door when the restrainer contained a cagemate. This pattern was observed regardless of whether rats were tested first with an empty restrainer (n=4; top) or with a trapped cagemate (n=5; bottom; P<0.001, mixed model analysis, PLSD). (B) In the chocolate experiment, rats (n=9) opened the door for a trapped cagemate as well as for a restrainer containing 5 chocolate chips. The median time to door-opening is shown across all days of testing. On days 6–12, rats opened both restrainers at similarly short latencies. (C) Rats in the chocolate empty condition (n=6) opened the empty restrainer at significantly longer latencies than the chocolate restrainer (P<0.01). The dashed lines in B–C indicate the time point when the door was opened half way by the experimenter.
Comment in
- Behavior. Empathy and the laws of affect.
Panksepp J. Panksepp J. Science. 2011 Dec 9;334(6061):1358-9. doi: 10.1126/science.1216480. Science. 2011. PMID: 22158811 No abstract available.
Similar articles
- Female rats release a trapped cagemate following shaping of the door opening response: Opening latency when the restrainer was baited with food, was empty, or contained a cagemate.
Blystad MH, Andersen D, Johansen EB. Blystad MH, et al. PLoS One. 2019 Oct 1;14(10):e0223039. doi: 10.1371/journal.pone.0223039. eCollection 2019. PLoS One. 2019. PMID: 31574116 Free PMC article. - Does a rat free a trapped rat due to empathy or for sociality?
Hachiga Y, Schwartz LP, Silberberg A, Kearns DN, Gomez M, Slotnick B. Hachiga Y, et al. J Exp Anal Behav. 2018 Sep;110(2):267-274. doi: 10.1002/jeab.464. Epub 2018 Jul 25. J Exp Anal Behav. 2018. PMID: 30047125 Free PMC article. - Anxiolytic Treatment Impairs Helping Behavior in Rats.
Ben-Ami Bartal I, Shan H, Molasky NM, Murray TM, Williams JZ, Decety J, Mason P. Ben-Ami Bartal I, et al. Front Psychol. 2016 Jun 8;7:850. doi: 10.3389/fpsyg.2016.00850. eCollection 2016. Front Psychol. 2016. PMID: 27375528 Free PMC article. - The complex affective and cognitive capacities of rats.
Ben-Ami Bartal I. Ben-Ami Bartal I. Science. 2024 Sep 20;385(6715):1298-1305. doi: 10.1126/science.adq6217. Epub 2024 Sep 19. Science. 2024. PMID: 39298607 Review. - The neuroscience of empathy and compassion in pro-social behavior.
Stevens F, Taber K. Stevens F, et al. Neuropsychologia. 2021 Aug 20;159:107925. doi: 10.1016/j.neuropsychologia.2021.107925. Epub 2021 Jun 26. Neuropsychologia. 2021. PMID: 34186105 Review.
Cited by
- Computational Transcendence: Responsibility and agency.
Deshmukh J, Srinivasa S. Deshmukh J, et al. Front Robot AI. 2022 Sep 26;9:977303. doi: 10.3389/frobt.2022.977303. eCollection 2022. Front Robot AI. 2022. PMID: 36226256 Free PMC article. - The fragrant power of collective fear.
Harb R, Taylor JR. Harb R, et al. PLoS One. 2015 May 6;10(5):e0123908. doi: 10.1371/journal.pone.0123908. eCollection 2015. PLoS One. 2015. PMID: 25945800 Free PMC article. - Interactive neurorobotics: Behavioral and neural dynamics of agent interactions.
Leonardis EJ, Breston L, Lucero-Moore R, Sena L, Kohli R, Schuster L, Barton-Gluzman L, Quinn LK, Wiles J, Chiba AA. Leonardis EJ, et al. Front Psychol. 2022 Aug 17;13:897603. doi: 10.3389/fpsyg.2022.897603. eCollection 2022. Front Psychol. 2022. PMID: 36059768 Free PMC article. - Dissecting social decision-making: A spotlight on oxytocinergic transmission.
Coccia G, La Greca F, Di Luca M, Scheggia D. Coccia G, et al. Front Mol Neurosci. 2022 Dec 22;15:1061934. doi: 10.3389/fnmol.2022.1061934. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36618824 Free PMC article. Review. - Affective mirror and anti-mirror neurons relate to prosocial help in rats.
Wu WY, Cheng Y, Liang KC, Lee RX, Yen CT. Wu WY, et al. iScience. 2022 Dec 23;26(1):105865. doi: 10.1016/j.isci.2022.105865. eCollection 2023 Jan 20. iScience. 2022. PMID: 36632059 Free PMC article.
References
- Batson DC. In: The Social Neuroscience of Empathy. Decety J, Ickes WJ, editors. Cambridge, MA: The MIT Press; 2009. pp. 3–15.
- Decety J, Jackson PL. Behav. Cogn Neurosci. Rev. 2004;3:71. - PubMed
- Eisenberg N, Miller PA, Fultz J, Shell F, et al. Journal of Personality and Social Psychology. 1989;57:55. - PubMed
- Materials and methods are available as supporting material on Science Online.
- Langford DJ, et al. Soc. Neurosci. 2010;5:163. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical