Rapid disruption of axon-glial integrity in response to mild cerebral hypoperfusion - PubMed (original) (raw)
. 2011 Dec 7;31(49):18185-94.
doi: 10.1523/JNEUROSCI.4936-11.2011.
Jamie McQueen, Luke Searcy, Gillian Scullion, Barbara Zonta, Anne Desmazieres, Philip R Holland, Jessica Smith, Catherine Gliddon, Emma R Wood, Pawel Herzyk, Peter J Brophy, James McCulloch, Karen Horsburgh
Affiliations
- PMID: 22159130
- PMCID: PMC4337974
- DOI: 10.1523/JNEUROSCI.4936-11.2011
Rapid disruption of axon-glial integrity in response to mild cerebral hypoperfusion
Michell M Reimer et al. J Neurosci. 2011.
Abstract
Myelinated axons have a distinct protein architecture essential for action potential propagation, neuronal communication, and maintaining cognitive function. Damage to myelinated axons, associated with cerebral hypoperfusion, contributes to age-related cognitive decline. We sought to determine early alterations in the protein architecture of myelinated axons and potential mechanisms after hypoperfusion. Using a mouse model of hypoperfusion, we assessed changes in proteins critical to the maintenance of paranodes, nodes of Ranvier, axon-glial integrity, axons, and myelin by confocal laser scanning microscopy. As early as 3 d after hypoperfusion, the paranodal septate-like junctions were damaged. This was marked by a progressive reduction of paranodal Neurofascin signal and a loss of septate-like junctions. Concurrent with paranodal disruption, there was a significant increase in nodal length, identified by Nav1.6 staining, with hypoperfusion. Disruption of axon-glial integrity was also determined after hypoperfusion by changes in the spatial distribution of myelin-associated glycoprotein staining. These nodal/paranodal changes were more pronounced after 1 month of hypoperfusion. In contrast, the nodal anchoring proteins AnkyrinG and Neurofascin 186 were unchanged and there were no overt changes in axonal and myelin integrity with hypoperfusion. A microarray analysis of white matter samples indicated that there were significant alterations in 129 genes. Subsequent analysis indicated alterations in biological pathways, including inflammatory responses, cytokine-cytokine receptor interactions, blood vessel development, and cell proliferation processes. Our results demonstrate that hypoperfusion leads to a rapid disruption of key proteins critical to the stability of the axon-glial connection that is mediated by a diversity of molecular events.
Figures
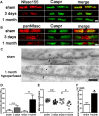
Figure 1.
Paranodal disruption occurs early in response to hypoperfusion in the corpus callosum. A, B, Colocalization of axonal Caspr and glial Neurofascin protein indicates intact septate-like junctions at the paranodes in the sham group. In response to hypoperfusion, at both 3 d and 1 month, there is a selective loss of Neurofascin colocalization with Caspr, which is indicative of a disruption of the paranodes. Scale bar, 1 μm. C, Electron micrographs show paranodal disruption in response to hypoperfusion in the optic nerve. Arrows indicate axonal membrane at the paranodal region; asterisks indicate paranodal loop. Disruption of septate-like junctions is indicated by loss of transverse bands. Scale bars, 0.1 μm. D, There is a nonsignificant increase in the number of paranodes disrupted at 3 d hypoperfusion (n = 5) compared with shams (n = 5), which is significant at 1 month after hypoperfusion (n = 4) compared with shams (n = 6). Analysis was conducted in the corpus callosum. *p < 0.05 (unpaired t test, two-tailed). E, The overlap coefficient after Manders, which is insensitive to differences in signal intensities between the two channels, shows significant loss in colocalization after 3 d and after 1 month of hypoperfusion (3 d sham: n = 13, hypoperfusion: n = 12; 1 month sham: n = 9, hypoperfusion: n = 9). Analysis was conducted in the corpus callosum. *p < 0.05, **p < 0.005 (unpaired t test, two-tailed). F, A significant increase in paranodes without septate-like junctions was observed after 1 month of hypoperfusion. *p < 0.05 (unpaired t test, two-tailed).
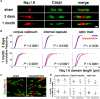
Figure 2.
Voltage-gated sodium channel distribution is rapidly disrupted by hypoperfusion. A, Nav1.6 sodium channels, which outline the node of Ranvier, are normally bounded by the paranodal protein Caspr, as shown in the sham. In response to hypoperfusion, after 3 d and 1 month, the distribution of the Nav1.6 sodium channels is markedly altered and laterally spreads along the axon (corpus callosum). Scale bar, 1 μm. B, There is a significant increase in the length of the Nav1.6 clusters after 3 d of hypoperfusion in the corpus callosum and, after 1 month, in the internal capsule and optic tract. Forty nodes per animal were analyzed and these 240–400 nodes are plotted as cumulative frequency distribution, tabulated as relative frequencies as percentage (two-sample Kolmogorov–Smirnov test). Number of nodes of Ranvier remains unchanged after 3 d or 1 month of hypoperfusion. C, Confocal stack of nodes of Ranvier in the corpus callosum, double-labeled with Nav1.6 and Caspr. Left, Sham; right, 1 month of hypoperfusion. Scale bar, 1 μm. D, Numbers of nodes of Ranvier are not altered in response to hypoperfusion. After 3 d and after 1 month, the number of Nav1.6 immunopositive nodes was not changed between the sham and hypoperfused groups. Analysis was conducted by confocal laser microscopy in the corpus callosum, internal capsule, and optic tract in a 47 × 47 × 5 μm3 confocal stack (Mann–Whitney, two-tailed).
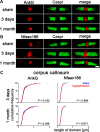
Figure 3.
AnkyrinG and Neurofascin186 distribution remains unchanged with hypoperfusion. A, Axonal AnkyrinG remains within the node of Ranvier in response to hypoperfusion. B, The spatial distribution of Neurofascin186, which connects nodal proteins to the extracellular matrix, does not change. Scale bar, 1 μm. C, There is no significant increase in the length of the AnkyrinG or Neurofascin186 domains after 3 d or 1 month of hypoperfusion in the corpus callosum. Forty nodes per animal were analyzed and these 160–400 nodes are plotted as cumulative frequency (two-sample Kolmogorov–Smirnov test).
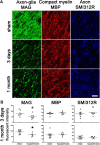
Figure 4.
Axon–glial integrity is disrupted, whereas myelin and axonal integrity remains intact. A, Disruption of axon–glial integrity was defined as reduced and discontinuous granular accumulation of the MAG staining in response to hypoperfusion compared with shams. In contrast, the integrity of the myelin sheath (assessed by MBP) and integrity of axons (assessed by SMI312R) remains intact at 3 d and at 1 month. Scale bar, 10 μm. B, Measurement of the fluorescent intensity of MAG, MBP, and SMI312R staining was conducted by confocal laser microscopy in the corpus callosum, internal capsule, and optic tract in sham and hypoperfused groups at 3 d (sham, n = 9; hypoperfused, n = 8) and 1 month (sham, n = 10; hypoperfused, n = 7). There was a significant reduction in the intensity of MAG staining at both 3 d and at 1 month hypoperfusion compared with shams. There were no alterations in MBP and SMI312R immunostaining in response to hypoperfusion at any time studied. *p < 0.05 (Mann–Whitney, two-tailed).
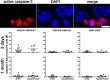
Figure 5.
Increased apoptosis does not occur after 3 d or 1 month of chronic cerebral hypoperfusion. The number of active caspase-3-positive cells in the corpus callosum, internal capsule, and optic tract is not increased in response to hypoperfusion. Colocalization of active caspase-3 with DAPI is shown. *p < 0.05 (Mann–Whitney, two-tailed).
Similar articles
- Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains.
Pillai AM, Thaxton C, Pribisko AL, Cheng JG, Dupree JL, Bhat MA. Pillai AM, et al. J Neurosci Res. 2009 Jun;87(8):1773-93. doi: 10.1002/jnr.22015. J Neurosci Res. 2009. PMID: 19185024 Free PMC article. - Reassembly of Excitable Domains after CNS Axon Regeneration.
Marin MA, de Lima S, Gilbert HY, Giger RJ, Benowitz L, Rasband MN. Marin MA, et al. J Neurosci. 2016 Aug 31;36(35):9148-60. doi: 10.1523/JNEUROSCI.1747-16.2016. J Neurosci. 2016. PMID: 27581456 Free PMC article. - Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier.
Rios JC, Rubin M, St Martin M, Downey RT, Einheber S, Rosenbluth J, Levinson SR, Bhat M, Salzer JL. Rios JC, et al. J Neurosci. 2003 Aug 6;23(18):7001-11. doi: 10.1523/JNEUROSCI.23-18-07001.2003. J Neurosci. 2003. PMID: 12904461 Free PMC article. - Molecular organization and function of vertebrate septate-like junctions.
Faivre-Sarrailh C. Faivre-Sarrailh C. Biochim Biophys Acta Biomembr. 2020 May 1;1862(5):183211. doi: 10.1016/j.bbamem.2020.183211. Epub 2020 Feb 4. Biochim Biophys Acta Biomembr. 2020. PMID: 32032590 Review. - Glial regulation of the axonal membrane at nodes of Ranvier.
Schafer DP, Rasband MN. Schafer DP, et al. Curr Opin Neurobiol. 2006 Oct;16(5):508-14. doi: 10.1016/j.conb.2006.08.003. Epub 2006 Sep 1. Curr Opin Neurobiol. 2006. PMID: 16945520 Review.
Cited by
- Modeling subcortical ischemic white matter injury in rodents: unmet need for a breakthrough in translational research.
Cui Y, Jin X, Choi JY, Kim BG. Cui Y, et al. Neural Regen Res. 2021 Apr;16(4):638-642. doi: 10.4103/1673-5374.295313. Neural Regen Res. 2021. PMID: 33063714 Free PMC article. - Impact of age-related neuroglial cell responses on hippocampal deterioration.
Ojo JO, Rezaie P, Gabbott PL, Stewart MG. Ojo JO, et al. Front Aging Neurosci. 2015 Apr 29;7:57. doi: 10.3389/fnagi.2015.00057. eCollection 2015. Front Aging Neurosci. 2015. PMID: 25972808 Free PMC article. Review. - Comparison of ion channel inhibitor combinations for limiting secondary degeneration following partial optic nerve transection.
Toomey LM, Bartlett CA, Majimbi M, Gopalasingam G, Rodger J, Fitzgerald M. Toomey LM, et al. Exp Brain Res. 2019 Jan;237(1):161-171. doi: 10.1007/s00221-018-5414-0. Epub 2018 Oct 26. Exp Brain Res. 2019. PMID: 30367192 - Life style, Perfusion deficits and Co-morbidities Precipitate Inflammation and Cerebrovascular Disorders in Aged Subjects.
Uzoni A, Ciobanu O, Sandu RE, Buga AM, Popa-Wagner A. Uzoni A, et al. Discoveries (Craiova). 2015 Mar 31;3(1):e39. doi: 10.15190/d.2015.31. Discoveries (Craiova). 2015. PMID: 32309564 Free PMC article. Review. - Metformin, Rapamycin, or Nicotinamide Mononucleotide Pretreatment Attenuate Cognitive Impairment After Cerebral Hypoperfusion by Inhibiting Microglial Phagocytosis.
Yu M, Zheng X, Cheng F, Shao B, Zhuge Q, Jin K. Yu M, et al. Front Neurol. 2022 Jun 13;13:903565. doi: 10.3389/fneur.2022.903565. eCollection 2022. Front Neurol. 2022. PMID: 35769369 Free PMC article.
References
- Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Brück W, Lucchinetti C, Schmidbauer M, Jellinger K, Lassmann H. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. - PubMed
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer's disease. Neurobiol Aging. 2004;25:843–851. - PubMed
- Bastin ME, Clayden JD, Pattie A, Gerrish IF, Wardlaw JM, Deary IJ. Diffusion tensor and magnetization transfer MRI measurements of periventricular white matter hyperintensities in old age. Neurobiol Aging. 2009;30:125–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous