Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads - PubMed (original) (raw)
Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads
Vipuil Kishore et al. Biomaterials. 2012 Mar.
Abstract
Topographical cues from the extracellular microenvironment can influence cellular activity including proliferation and differentiation. Information on the effects of material topography on tenogenic differentiation of human mesenchymal stem cells (human MSCs) is limited. A methodology using the principles of isoelectric focusing has previously been developed in our laboratory to synthesize electrochemically aligned collagen (ELAC) threads that mimics the packing density, alignment and strength of collagen dense connective tissues. In the current study, human MSCs were cultured on ELAC and randomly oriented collagen threads and the effect of collagen orientation on cell morphology, proliferation and tenogenic differentiation was investigated. The results indicate that higher rates of proliferation were observed on randomly oriented collagen threads compared to ELAC threads. On the other hand, tendon specific markers such as scleraxis and tenomodulin, were significantly increased on ELAC threads compared to randomly oriented collagen threads. Additionally, osteocalcin, a specific marker of bone differentiation was suppressed on ELAC threads. Previous studies have reported that BMP-12 is a key growth factor to induce tenogenic differentiation of MSCs. To evaluate the synergistic effect of BMP-12 and collagen orientation, human MSCs were cultured on ELAC threads in culture medium supplemented with and without BMP-12. The results revealed that BMP-12 did not have an additional effect on the tenogenic differentiation of human MSCs on ELAC threads. Together, these results suggest that ELAC induces tenogenic differentiation of human MSCs by presenting an aligned and dense collagen substrate, akin to the tendon itself. In conclusion, ELAC has a significant potential to be used as a tendon replacement and in the development of an osteotendinous construct towards the regeneration of bone-tendon interfaces.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
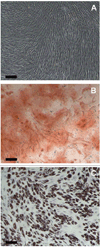
Figure 1
Validation of the Stemness of Human MSCs. (A) Day 14 – Control (No induction), (B) Day 14 - Alizarin red S staining after induction of osteogenic differentiation of human MSCs, (C) Day 14 – Oil red O staining after induction of adipogenic differentiation of human MSCs. Scale Bar: 200 µm.
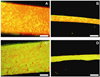
Figure 2
Visual Examination of Cell Morphology and Distribution by Actin Staining. Actin stained images of Random thread at day 1 (A), ELAC thread at day 1 (B), Random thread at day 14 (C), ELAC thread at day 14 (D). Scale Bar: 0.5 mm.
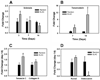
Figure 3
Effect of Collagen Orientation on Cell Adhesion and Proliferation. (A) Cell Adhesion - Cell adhesion was two-fold higher on ELAC threads compared to random collagen threads. (B) Cell Proliferation – Higher rates of cell proliferation was observed on random collagen threads compared to ELAC threads. (* indicates statistical significance between ELAC and random collagen threads).
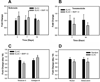
Figure 4
Effect of Collagen Orientation on Tenogenic Differentiation of Human MSCs. (A) Scleraxis, (B) Tenomodulin, (C) Tendon ECM Genes (Tenascin-C and Collagen-III), (D) Bone Differentiation Markers (Runx2 and Osteocalcin). Increase in the expression of tendon specific (scleraxis and tenomodulin) and tendon related (tenascin-D and collagen-III) genes indicates that the oriented collagen topography of ELAC threads stimulates the tenogenic differentiation of human MSCs. Data are normalized to β-actin and relative to the random collagen control. . (* indicates statistical significance between ELAC and random collagen threads).
Figure 5
Effect of Collagen Orientation on Cell Morphology, Alignment and ECM Deposition by Scanning Electron Microscopy. (A) Random – day 2, (B) ELAC – day 2, (C) Random – day 14, (D) ELAC – day 14, (E) Random – day 14 (High Mag), (F) ELAC – day 14 (High Mag). Cells have been highlighted in blue for clarity.
Figure 6
Effect of BMP-12 on Tenogenic Differentiation of Human MSCs on ELAC Threads. . (A) Scleraxis, (B) Tenomodulin, (C) Tendon ECM Genes (Tenascin-C and Collagen-III), (D) Bone Differentiation Markers (Runx2 and Osteocalcin). The expression of tendon specific genes (scleraxis and tenomodulin) was comparable with or without BMP-12 treatment indicating that BMP-12 did not have an additive effect over collagen orientation on the tenogenic differentiation of human MSCs. Data are normalized to β-actin and relative to ELAC without BMP-12 control. (* indicates statistical significance between ELAC and random collagen threads).
Similar articles
- Effects of phosphate-buffered saline concentration and incubation time on the mechanical and structural properties of electrochemically aligned collagen threads.
Uquillas JA, Kishore V, Akkus O. Uquillas JA, et al. Biomed Mater. 2011 Jun;6(3):035008. doi: 10.1088/1748-6041/6/3/035008. Epub 2011 May 4. Biomed Mater. 2011. PMID: 21540522 Free PMC article. - Bioglass incorporation improves mechanical properties and enhances cell-mediated mineralization on electrochemically aligned collagen threads.
Nijsure MP, Pastakia M, Spano J, Fenn MB, Kishore V. Nijsure MP, et al. J Biomed Mater Res A. 2017 Sep;105(9):2429-2440. doi: 10.1002/jbm.a.36102. Epub 2017 May 22. J Biomed Mater Res A. 2017. PMID: 28470671 - Tendon Tissue Engineering: Effects of Mechanical and Biochemical Stimulation on Stem Cell Alignment on Cell-Laden Hydrogel Yarns.
Rinoldi C, Costantini M, Kijeńska-Gawrońska E, Testa S, Fornetti E, Heljak M, Ćwiklińska M, Buda R, Baldi J, Cannata S, Guzowski J, Gargioli C, Khademhosseini A, Swieszkowski W. Rinoldi C, et al. Adv Healthc Mater. 2019 Apr;8(7):e1801218. doi: 10.1002/adhm.201801218. Epub 2019 Feb 6. Adv Healthc Mater. 2019. PMID: 30725521 - Tendon tissue engineering: Current progress towards an optimized tenogenic differentiation protocol for human stem cells.
Donderwinkel I, Tuan RS, Cameron NR, Frith JE. Donderwinkel I, et al. Acta Biomater. 2022 Jun;145:25-42. doi: 10.1016/j.actbio.2022.04.028. Epub 2022 Apr 22. Acta Biomater. 2022. PMID: 35470075 Review. - Biomaterial Properties and Differentiation Strategies for Tenogenic Differentiation of Mesenchymal Stem Cells.
Roets B, Abrahamse H, Crous A. Roets B, et al. Cells. 2025 Mar 18;14(6):452. doi: 10.3390/cells14060452. Cells. 2025. PMID: 40136701 Free PMC article. Review.
Cited by
- Computational and Experimental Characterization of Aligned Collagen across Varied Crosslinking Degrees.
Lin S, Patrawalla NY, Zhai Y, Dong P, Kishore V, Gu L. Lin S, et al. Micromachines (Basel). 2024 Jun 29;15(7):851. doi: 10.3390/mi15070851. Micromachines (Basel). 2024. PMID: 39064362 Free PMC article. - Physical and Soluble Cues Enhance Tendon Progenitor Cell Invasion into Injectable Synthetic Hydrogels.
Kent RN 3rd, Said M, Busch ME, Poupard ER, Tsai A, Xia J, Matera DL, Wang WY, DePalma SJ, Hiraki HL, Killian ML, Abraham AC, Shin JW, Huang AH, Shikanov A, Baker BM. Kent RN 3rd, et al. Adv Funct Mater. 2022 Nov 24;32(48):2207556. doi: 10.1002/adfm.202207556. Epub 2022 Sep 28. Adv Funct Mater. 2022. PMID: 39257859 Free PMC article. - Collagen Substrate Stiffness Anisotropy Affects Cellular Elongation, Nuclear Shape, and Stem Cell Fate toward Anisotropic Tissue Lineage.
Islam A, Younesi M, Mbimba T, Akkus O. Islam A, et al. Adv Healthc Mater. 2016 Sep;5(17):2237-47. doi: 10.1002/adhm.201600284. Epub 2016 Jul 5. Adv Healthc Mater. 2016. PMID: 27377355 Free PMC article. - Stem cell sheet interpositioned between the tendon and bone would be better for healing than stem cell sheet overlaid above the tendon-to-bone junction in rotator cuff repair of rats.
Choi JH, Shim IK, Shin MJ, Lee YN, Koh KH. Choi JH, et al. PLoS One. 2022 Mar 24;17(3):e0266030. doi: 10.1371/journal.pone.0266030. eCollection 2022. PLoS One. 2022. PMID: 35324992 Free PMC article. - Rho/ROCK Inhibition Promotes TGF-_β_3-Induced Tenogenic Differentiation in Mesenchymal Stromal Cells.
Melzer M, Schubert S, Müller SF, Geyer J, Hagen A, Niebert S, Burk J. Melzer M, et al. Stem Cells Int. 2021 Oct 8;2021:8284690. doi: 10.1155/2021/8284690. eCollection 2021. Stem Cells Int. 2021. PMID: 34659420 Free PMC article.
References
- Squier CA, Magnes C. Spatial relationships between fibroblasts during the growth of rat-tail tendon. Cell Tissue Res. 1983;234:17–29. - PubMed
- Fischer LP, Carret JP, Gonon GP, Sayfi Y. Arterial vascularization of the patellar ligament (ligamentum patellase) and of the Achilles tendon (tendo calcaneous) in man. Bull Assoc Anat (Nancy) 1976;60:323–334. - PubMed
- Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11:909–917. - PubMed
- Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17:971–980. - PubMed
- Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, et al. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources