Valproic acid improves locomotion in vivo after SCI and axonal growth of neurons in vitro - PubMed (original) (raw)
Comparative Study
Valproic acid improves locomotion in vivo after SCI and axonal growth of neurons in vitro
Lei Lv et al. Exp Neurol. 2012 Feb.
Abstract
Previous studies have found that valproic acid (VPA), a histone deacetylases (HDAC) inhibitor, improves outcomes in a rat model of spinal cord injury (SCI). The study here aimed to further illuminate the neuroprotective effects of VPA against SCI, both in vivo and in vitro. First, spinal cord injury was performed in rats using NYU impactor. Delayed VPA injection (8 h following SCI) significantly accelerated locomotor recovery. VPA therapy also suppressed SCI-induced hypoacetylation of histone and promoted expressions of BDNF and GDNF. Next, the influence of VPA on axonal growth inhibited by a myelin protein was tested. Neurons from embryonic spinal cord or hippocampus were cultured on plates coated with Nogo-A peptide, and escalating concentrations of VPA were added into the cultures. VPA treatment, in a concentration dependent manner, allowed neurons to overcome Nogo-A inhibition of neurite outgrowth. Meanwhile, VPA exposure increased the level of histone acetylation and expression of BDNF in spinal neurons. Cumulatively, these findings indicate that VPA is possibly a promising medication and deserves translational trials for spinal cord injury.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Statement of interest
None.
Figures
Fig. 1
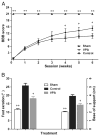
Delayed administration of VPA improved functional recovery. SCI rats were treated by VPA or saline, staring 8 h after surgery. Compared to the controls, VPA significantly accelerated locomotion recovery after SCI. (A) The rats treated by VPA had higher scores in BBB test, starting from week 4 and lasting to endpoint. (B) At week 6, SCI rats injected by VPA showed better performance in footprint analysis. Reduced foot exorotation and support were found in animals with VPA-injection. Data were presented as mean±SEM (n=12), and p<0.05*, p<0.01**.
Fig. 2
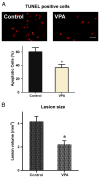
Delayed VPA treatment reduced apoptotic cells and lesion size. (A) Apoptotic cells were detected by TUNEL kit week 1 post operative. Delayed VPA treatment significantly decreased apoptotic cells. (B) Lesion volume was measured 6 weeks after injury. Delayed VPA treatment also significantly reduced lesion size. Data were presented as means±SEM (n=8), Bar=20 μm, and p<0.05*, as compared to controls.
Fig. 3
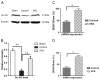
Delayed VPA therapy unregulated H3 acetylation, and mRNA expressions of BDNF and GDNF. Rats were sacrificed to investigate acetylation of histone and expressions of neurotrophic genes. (A/B) VPA injection ameliorated SCI-induced hypoacetylation of H3. (C/D) The mRNA expressions of BDNF and GDNF were also elevated by VPA therapy. Data were presented as means±SEM (n=6), and p<0.05*, p<0.01**.
Fig. 4
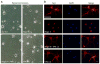
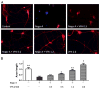
VPA attenuated Nogo-A inhibition on axonal growth of spinal neurons. (A) Spinal neurons were cultured on 24-well coated with or without Nogo-A peptide. Compared to control, Nogo-A inhibited axonal growth. However, VPA exposure attenuated Nogo-A inhibition and promoted neurite outgrowth. (B) Neurons were immunostained with antibodies to β-III tubulin to trace neurite (red), and nuclei were stained by DAPI (blue). VPA obviously enhanced neurite generation. Bar A=40 μm, Bar B=100 μm.
Fig. 5
VPA enhances axon outgrowth in a concentration dependent manner. (A) Spinal neurons were stained with antibodies to β-III tubulin (red) and digital images were taken for morphometrical analysis. (B) The length of axon was determined and average outgrowth was calculated. VPA attenuated the inhibitory effect of Nogo-A and enhanced neurite regeneration in a concentration dependent manner. Neurite outgrowth was expressed as a ratio to control and presented as mean±SEM (n=3). Bar=100 μm, and p<0.05*, p<0.01** as compared to Nogo-A group; p<0.05# as compared to control group.
Fig. 6
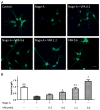
VPA enhances axonal growth of hippocampus neurons. (A) Immunostaining of β-III tubulin (green) was performed in hippocampus neurons. (B) The average outgrowth of neurite was analyzed. VPA in a concentration dependent manner enhanced axonal regeneration. Neurite outgrowth was expressed as a ratio to control and presented as mean± SEM (n=3). Bar=100 μm, and p<0.05*, p<0.01** as compared to Nogo-A group; p<0.05# as compared to control group.
Fig. 7
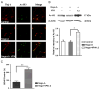
VPA enhanced H3 acetylating and BDNF transcript in spinal neurons. (A) Neurons were immunostained with antibodies to β-III tubulin (red) and acetylated histone 3 (green). Compared to controls, VPA (1.2 mM) increased Ac-H3. (B) Weston blot was performed to quantify Ac-H3. VPA significantly enhanced H3 acetylating in neurons attained from spinal cord. (C) The level of BDNF mRNA in spinal cord neurons was measured by q-PCR. Compared to controls, VPA significantly upregulated BDNF transcript. Data were presented as means±SEM (n=3), Bar=100 μm, p<0.05* and p<0.01**.
Similar articles
- Valproic acid: a new candidate of therapeutic application for the acute central nervous system injuries.
Chen S, Wu H, Klebe D, Hong Y, Zhang J. Chen S, et al. Neurochem Res. 2014 Sep;39(9):1621-33. doi: 10.1007/s11064-014-1241-2. Epub 2014 Jan 31. Neurochem Res. 2014. PMID: 24482021 Review. - Valproic acid improves outcome after rodent spinal cord injury: potential roles of histone deacetylase inhibition.
Lv L, Sun Y, Han X, Xu CC, Tang YP, Dong Q. Lv L, et al. Brain Res. 2011 Jun 17;1396:60-8. doi: 10.1016/j.brainres.2011.03.040. Epub 2011 Mar 22. Brain Res. 2011. PMID: 21439269 - Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3.
Chen S, Ye J, Chen X, Shi J, Wu W, Lin W, Lin W, Li Y, Fu H, Li S. Chen S, et al. J Neuroinflammation. 2018 May 18;15(1):150. doi: 10.1186/s12974-018-1193-6. J Neuroinflammation. 2018. PMID: 29776446 Free PMC article. - Valproic acid attenuates microgliosis in injured spinal cord and purinergic P2X4 receptor expression in activated microglia.
Lu WH, Wang CY, Chen PS, Wang JW, Chuang DM, Yang CS, Tzeng SF. Lu WH, et al. J Neurosci Res. 2013 May;91(5):694-705. doi: 10.1002/jnr.23200. Epub 2013 Feb 13. J Neurosci Res. 2013. PMID: 23404572 Free PMC article. - Effects of Valproic Acid Therapy on Rats with Spinal Cord Injury: A Systematic Review and Meta-Analysis.
Yang Q, Zhang H, Jin Z, Zhang B, Wang Y. Yang Q, et al. World Neurosurg. 2024 Feb;182:12-28. doi: 10.1016/j.wneu.2023.10.135. Epub 2023 Nov 3. World Neurosurg. 2024. PMID: 37923014 Review.
Cited by
- Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder.
Chiu CT, Wang Z, Hunsberger JG, Chuang DM. Chiu CT, et al. Pharmacol Rev. 2013 Jan 8;65(1):105-42. doi: 10.1124/pr.111.005512. Print 2013 Jan. Pharmacol Rev. 2013. PMID: 23300133 Free PMC article. Review. - Valproic acid: a new candidate of therapeutic application for the acute central nervous system injuries.
Chen S, Wu H, Klebe D, Hong Y, Zhang J. Chen S, et al. Neurochem Res. 2014 Sep;39(9):1621-33. doi: 10.1007/s11064-014-1241-2. Epub 2014 Jan 31. Neurochem Res. 2014. PMID: 24482021 Review. - Epigenetic regulation of sensory axon regeneration after spinal cord injury.
Finelli MJ, Wong JK, Zou H. Finelli MJ, et al. J Neurosci. 2013 Dec 11;33(50):19664-76. doi: 10.1523/JNEUROSCI.0589-13.2013. J Neurosci. 2013. PMID: 24336730 Free PMC article. - Advances and Limitations of Current Epigenetic Studies Investigating Mammalian Axonal Regeneration.
Palmisano I, Di Giovanni S. Palmisano I, et al. Neurotherapeutics. 2018 Jul;15(3):529-540. doi: 10.1007/s13311-018-0636-1. Neurotherapeutics. 2018. PMID: 29948919 Free PMC article. Review. - Decoding epigenetic codes: new frontiers in exploring recovery from spinal cord injury.
Zhang BY, Chang PY, Zhu QS, Zhu YH; Saijilafu. Zhang BY, et al. Neural Regen Res. 2020 Sep;15(9):1613-1622. doi: 10.4103/1673-5374.276323. Neural Regen Res. 2020. PMID: 32209760 Free PMC article. Review.
References
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. - PubMed
- Biermann J, Grieshaber P, Goebel U, Martin G, Thanos S, Di Giovanni S, Lagreze WA. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Investig Ophthalmol Vis Sci. 2010;51:526–534. - PubMed
- Boato F, Hendrix S, Huelsenbeck SC, Hofmann F, Grosse G, Djalali S, Klimaschewski L, Auer M, Just I, Ahnert-Hilger G, Holtje M. C3 peptide enhances recovery from spinal cord injury by improved regenerative growth of descending fiber tracts. J Cell Sci. 2010;123:1652–1662. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical