Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle - PubMed (original) (raw)
Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle
Julia von Maltzahn et al. Nat Cell Biol. 2011.
Abstract
Wnt7a signals through its receptor Fzd7 to activate the planar-cell-polarity pathway and drive the symmetric expansion of satellite stem cells resulting in enhanced repair of skeletal muscle. In differentiated myofibres, we observed that Wnt7a binding to Fzd7 directly activates the Akt/mTOR growth pathway, thereby inducing myofibre hypertrophy. Notably, the Fzd7 receptor complex was associated with Gα(s) and PI(3)K and these components were required for Wnt7a to activate the Akt/mTOR growth pathway in myotubes. Wnt7a-Fzd7 activation of this pathway was completely independent of IGF-receptor activation. Together, these experiments demonstrate that Wnt7a-Fzd7 activates distinct pathways at different developmental stages during myogenic lineage progression, and identify a non-canonical anabolic signalling pathway for Wnt7a and its receptor Fzd7 in skeletal muscle.
Conflict of interest statement
COMPETING FINANCIAL INTEREST
The authors declare a competing financial interest. M.A.R. is a founding scientist and consultant with Fate Therapeutics.
Figures
Figure 1
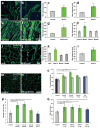
Wnt7a induces hypertrophy in differentiated myotubes and myofibres. (a, b) Primary myoblasts derived from satellite cells were differentiated for 5 days in medium containing 50 ng/ml Wnt7a recombinant protein or BSA as a control. Staining for myosin heavy chain (MyHC, in green) marks differentiated cells. (c) Quantification of the fibre diameter as in a, b. n=3 (d) Quantification of the fibre diameter of C2C12 cells treated with 50 ng/ml recombinant Wnt7a protein. The Wnt7a recombinant protein was applied at day 3 of differentiation when the majority of the cells are already differentiated. n=3 (e,f) C2C12 cells were stably transfected with a CMV-Wnt7a-HA expression plasmid and differentiated for 5 days. (g) Quantification of the fibre diameter as in e,f. n=3 (h) Application of different recombinant Wnt proteins (50 ng/ml each) revealed that induction of hypertrophy is a Wnt7a specific phenomenon. n=3 (i,j) Electroporation of the CMV-Wnt7a-HA expression plasmid (40 μg) into the tibialis anterior muscle of adult mice resulted in increased fibre diameters 2 weeks after electroporation compared to a control (CMV-lacZ) plasmid. (k) Quantification of the fibre diameter of TA muscles electroporated with expression plasmids for Wnt3a-HA, Wnt5a-HA, Wnt7a-HA or a lacZ control plasmid (40 ug plasmid each). n=4 (l) Quantification of a single injection of recombinant Wnt7a protein (2.5 ug) into the tibialis anterior muscle of adult mice, mice were sacrificed two weeks after injection. n=4 (m,n) representative images of immunostained sections of TA muscle showing Pax7- (red) and laminin-staining (green). Nuclei were counterstained with Dapi (blue) (o) Intramuscular injection of recombinant Wnt7a protein into the tibialis anterior (TA) muscle resulted in a significant increase in the muscle weight, without affecting the weight of the contralateral muscle three weeks after injection. Injection of long-IGF stimulated an equal increase in both injected and contralateral TA muscle. n=4 (p) Wnt7a stimulated an expansion in the satellite cell pool whereas IGF did not. n=4 (q) Wnt7a and IGF injection resulted in a significant increase in fibre calibre. Grey bars indicate the contralateral muscle.. n=4, *p<0.01, **p<0.001, *** p<0.0001. Error bars represent SEM.
Figure 2
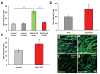
Wnt7a induces muscle hypertrophy through its receptor Fzd7. (a) siRNA mediated knockdown of Fzd7 inhibited the induction of hypertrophy in Wnt7a-HA expressing myotubes. Expression of Fzd7YFP is sufficient to induce significant hypertrophy in (b) differentiated C2C12 cells and (c) differentiated primary myoblasts. (d,e) Representative images of C2C12 cells expressing Fzd7-YFP or a control plasmid after 5 days of differentiation. Staining for myosin heavy chain (MyHC, in green) marks differentiated cells. Nuclei are counterstained with Dapi (blue). (f,g) Representative images of differentiated primary myoblasts expressing Fzd7-YFP or a control plasmid. Differentiation was carried out for 5 days. Scale bar: 50 μm (n=3), *** p<0.0001. Error bars represent SEM
Figure 3
Wnt7a activates the Akt/mTOR pathway in differentiated myotubes and myofibres. (a) Immunoblot analyses revealed increased phosphorylation of members of the Akt/mTOR pathway in C2C12 expressing Wnt7a-HA compared to control cells. Members of other known non-canonical pathway as PKC, CamKII or c-jun, a downstream effector of JNK, are not activated in Wnt7a-HA expressing myotubes. Increased levels of phosphorylated Foxo1/3a were observed in Wnt7a-HA expressing cells as well as increased levels of total Foxo1 in myoblasts and myotubes and Foxo3a in myoblasts. n=3. (b) Injection of Wnt7a recombinant protein into tibialis anterior muscle of adult mice leads to an activation of the Akt/mTOR pathway as visualized by increased phosphorylation of S6 and Akt. n=3. (c) Application of 50 ng/ml Wnt7a recombinant protein to differentiated C2C12 cells (5 days differentiation) resulted in increased levels of phosphorylated Akt and phosphorylated S6 demonstrating the rapid activation of the Akt/mTOR pathway by Wnt7a. n=3. (d) Inhibition of mTOR by rapamycin abolished hypertrophy in Wnt7a-HA expressing C2C12 cells, the fibre diameter was measured 5 days after induction of differentiation (n=4), *** p<0.001. Error bars represent SEM. See S6 for uncropped images of blots.
Figure 4
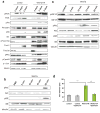
Wnt7a induces hypertrophy independent of IGF-receptor activity. (a) Immunoblot analyses revealed no changes in the phosphorylation of IGF receptor as well as IRS-1 in Wnt7a-HA expressing cells compared to control cells. Additionally the total amount of IGF-receptor and IRS1 were not significantly changed in Wnt7a-HA expressing cells compared to control cells. n=3. (b) Inhibition of IGF receptor activity by AG1024 did not result in changes in fibre diameter in Wnt7a-HA expressing cells suggesting that Wnt7a acts independently of IGF receptor activity. All analyses were performed in cells which were differentiated for 5 days. n=3. (c) Quantitative Real-time PCR of members of the IGF and IGF receptor family (n=3), *** p<0.0001. Error bars represent SEM. See S6 for uncropped images of blots.
Figure 5
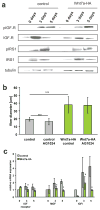
The Fzd7 receptor complex contains PI3K and the G protein Gαs (a) Coimmunoprecipitation analyses demonstrated that Fzd7YFP binds the p85 subunit of PI3K in myotubes but not in myoblasts. (b) Blocking of PI3K activity with the specific inhibitor LY294002 abrogated the hypertrophy induced by Wnt7a. Fibre diameter was measured 5 days after induction of differentiation in C2C12 control cells as well as in C2C12 cells expressing Wnt7a-HA (n=4), *** p<0.001. (c) IP-Western coimmunoprecipitation analysis demonstrated that Fzd7 binds G protein Gαs. (d). Suramin, a specific inhibitor of the G protein Gαs completely blocked Wnt7a induced hypertrophy, n=3, *** p<0.001. (e) Suramin inhibits Wnt7a induced phosphorylation of Akt and S6. (f) Knockdown of GNAS1 abrogates Wnt7a induced hypertrophy in C2C12 cells, n=3, *** p<0.001. Error bars represent SEM. See S6 for uncropped images of blots.
Similar articles
- Wnt7a stimulates myogenic stem cell motility and engraftment resulting in improved muscle strength.
Bentzinger CF, von Maltzahn J, Dumont NA, Stark DA, Wang YX, Nhan K, Frenette J, Cornelison DD, Rudnicki MA. Bentzinger CF, et al. J Cell Biol. 2014 Apr 14;205(1):97-111. doi: 10.1083/jcb.201310035. Epub 2014 Apr 7. J Cell Biol. 2014. PMID: 24711502 Free PMC article. - A truncated Wnt7a retains full biological activity in skeletal muscle.
von Maltzahn J, Zinoviev R, Chang NC, Bentzinger CF, Rudnicki MA. von Maltzahn J, et al. Nat Commun. 2013;4:2869. doi: 10.1038/ncomms3869. Nat Commun. 2013. PMID: 24287629 Free PMC article. - Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells.
Le Grand F, Jones AE, Seale V, Scimè A, Rudnicki MA. Le Grand F, et al. Cell Stem Cell. 2009 Jun 5;4(6):535-47. doi: 10.1016/j.stem.2009.03.013. Cell Stem Cell. 2009. PMID: 19497282 Free PMC article. - Influence of Age on Skeletal Muscle Hypertrophy and Atrophy Signaling: Established Paradigms and Unexpected Links.
Lee EJ, Neppl RL. Lee EJ, et al. Genes (Basel). 2021 May 3;12(5):688. doi: 10.3390/genes12050688. Genes (Basel). 2021. PMID: 34063658 Free PMC article. Review. - PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.
Glass DJ. Glass DJ. Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
Cited by
- Wnt7a is Required for Regeneration of Dystrophic Skeletal Muscle.
Gurriaran-Rodriguez U, Kodippili K, Datzkiw D, Javandoost E, Xiao F, Rejas MT, Rudnicki MA. Gurriaran-Rodriguez U, et al. bioRxiv [Preprint]. 2024 Jan 25:2024.01.24.577041. doi: 10.1101/2024.01.24.577041. bioRxiv. 2024. PMID: 38328077 Free PMC article. Updated. Preprint. - The hairpin region of WNT7A is sufficient for binding to the Frizzled7 receptor and to elicit signaling in myogenic cells.
Schmidt M, Poser C, Janster C, von Maltzahn J. Schmidt M, et al. Comput Struct Biotechnol J. 2022 Nov 12;20:6348-6359. doi: 10.1016/j.csbj.2022.10.047. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36420144 Free PMC article. - Intrafusal-fiber LRP4 for muscle spindle formation and maintenance in adult and aged animals.
Cao R, Chen P, Wang H, Jing H, Zhang H, Xing G, Luo B, Pan J, Yu Z, Xiong WC, Mei L. Cao R, et al. Nat Commun. 2023 Feb 10;14(1):744. doi: 10.1038/s41467-023-36454-8. Nat Commun. 2023. PMID: 36765071 Free PMC article. - Wnt and extraocular muscle sparing in amyotrophic lateral sclerosis.
McLoon LK, Harandi VM, Brännström T, Andersen PM, Liu JX. McLoon LK, et al. Invest Ophthalmol Vis Sci. 2014 Aug 14;55(9):5482-96. doi: 10.1167/iovs.14-14886. Invest Ophthalmol Vis Sci. 2014. PMID: 25125606 Free PMC article. - Hypoxia with or without Treadmill Exercises Affects Slow-Twitch Muscle Atrophy and Joint Destruction in a Rat Model of Rheumatoid Arthritis.
Kamada Y, Arai Y, Toyama S, Inoue A, Nakagawa S, Fujii Y, Kaihara K, Cha R, Mazda O, Takahashi K. Kamada Y, et al. Int J Mol Sci. 2023 Jun 5;24(11):9761. doi: 10.3390/ijms24119761. Int J Mol Sci. 2023. PMID: 37298711 Free PMC article.
References
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. The Journal of biological chemistry. 2006;281:22429–22433. - PubMed
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annual review of cell and developmental biology. 1998;14:59–88. - PubMed
- Tajbakhsh S, et al. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development (Cambridge, England) 1998;125:4155–4162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous