Activation of intracellular metabotropic glutamate receptor 5 in striatal neurons leads to up-regulation of genes associated with sustained synaptic transmission including Arc/Arg3.1 protein - PubMed (original) (raw)
Activation of intracellular metabotropic glutamate receptor 5 in striatal neurons leads to up-regulation of genes associated with sustained synaptic transmission including Arc/Arg3.1 protein
Vikas Kumar et al. J Biol Chem. 2012.
Abstract
The G-protein coupled receptor, metabotropic glutamate receptor 5 (mGluR5), is expressed on both cell surface and intracellular membranes in striatal neurons. Using pharmacological tools to differentiate membrane responses, we previously demonstrated that cell surface mGluR5 triggers rapid, transient cytoplasmic Ca(2+) rises, resulting in c-Jun N-terminal kinase, Ca(2+)/calmodulin-dependent protein kinase, and cyclic adenosine 3',5'-monophosphate-responsive element-binding protein (CREB) phosphorylation, whereas stimulation of intracellular mGluR5 induces long, sustained Ca(2+) responses leading to the phosphorylation of extracellular signal-regulated kinase (ERK1/2) and Elk-1 (Jong, Y. J., Kumar, V., and O'Malley, K. L. (2009) J. Biol. Chem. 284, 35827-35838). Using pharmacological, genetic, and bioinformatics approaches, the current findings show that both receptor populations up-regulate many immediate early genes involved in growth and differentiation. Activation of intracellular mGluR5 also up-regulates genes involved in synaptic plasticity including activity-regulated cytoskeletal-associated protein (Arc/Arg3.1). Mechanistically, intracellular mGluR5-mediated Arc induction is dependent upon extracellular and intracellular Ca(2+) and ERK1/2 as well as calmodulin-dependent kinases as known chelators, inhibitors, and a dominant negative Ca(2+)/calmodulin-dependent protein kinase II construct block Arc increases. Moreover, intracellular mGluR5-induced Arc expression requires the serum response transcription factor (SRF) as wild type but not SRF-deficient neurons show this response. Finally, increased Arc levels due to high K(+) depolarization is significantly reduced in response to a permeable but not an impermeable mGluR5 antagonist. Taken together, these data highlight the importance of intracellular mGluR5 in the cascade of events associated with sustained synaptic transmission.
Figures
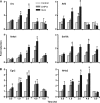
FIGURE 1.
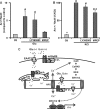
Microarray analysis reveals both common and distinct transcriptional changes induced by Quis and DHPG. Striatal neurons were treated with Quis or DHPG for 1 h, and total RNA was isolated, further processed, and used for Affymetrix Rat 230V2 gene chip hybridization. Data were analyzed as described under “Experimental Procedures.” A, shown is the number of genes with increased expression in both treatments. B, shown are genes that were specifically up-regulated or down-regulated by Quis treatment but not DHPG. Bars represents -fold change as compared with the control (n = 3). C and D, pie charts show the cellular localization and molecular function distribution, respectively, of 12 known genes specifically up-regulated by Quis treatment but not by DHPG.
FIGURE 2.
Quis-specific up-regulated genes validated by quantitative RT-PCR. Striatal neurons were treated with Quis (black) or DHPG (gray) for indicated time points, total RNA was isolated, and the expression levels of genes by Quis only (A) or both DHPG and Quis (B) were measured by quantitative reverse transcriptase-PCR normalized to the expression levels of the reference gene, Gapdh. Bars represent fold change compared with control (mean ± S.E.) from at least three independent experiments performed in triplicate. *, p < 0.05 versus control.
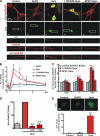
FIGURE 3.
Activation of intracellular, but not cell surface mGluR5, up-regulates immediate early gene Arc. A, DIV17–18 striatal neurons were treated with the indicated drugs for 3 h before staining for Arc (green) and mGluR5 (red). Quis but not DHPG increased Arc expression in mGluR5-positive cell bodies and neurites. MPEP (10 μ
m
) but not LY393053 (20 μ
m
) abolished Quis-induced Arc levels. B, quantitation of Arc immunofluorescence in neurons (expressed as mean ± S.E., compared with control) in the absence or presence of various ligands treated for indicated times; more than 2500 neurons per treatment condition from 3–6 independent experiments were assessed using High Content Imaging; *, p < 0.05 versus basal level. C, quantitation of Arc staining intensity in mGluR5-positive neurites was measured up to 50 μm from the cell body. Bars represent the mean ± S.E. from at least three independent experiments with more than 140 neurites per treatment condition analyzed. *, p < 0.05; **, p < 0.001 versus basal levels. D, Quis-mediated Arc induction is dependent on transcription and translation. Quantitation of Arc immunofluorescence in neurons (expressed as % of Quis treatment) in the absence or presence of the indicated antagonists (ActD, actinomycin D, transcription inhibitor, 40 μ
m
; CHX, cycloheximide, translation inhibitor, 80 μ
m
) treated for 1 h; ∼700 neurons per treatment condition from n = 4 independent experiments. **, p < 0.001 _versus_ Quis. _E_, Quis but not DHPG induced Arc+ nuclear puncta. _Bars_ represent mean ± S.E. from three independent experiments: >200 neurons were analyzed per treatment. **, p < 0.05.
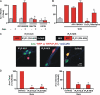
FIGURE 4.
Quis-mediated Arc induction is dependent on Ca2+. A, striatal neurons were treated with Quis (red) in the absence or presence of indicated antagonists (GF109203X, protein kinase C inhibitor, 1 μ
m
; BAPTA-1AM, intracellular Ca2+ chelator, 30 μ
m
; EGTA, extracellular Ca2+ chelator, 5 m
m
) for 1 h, fixed, and stained for Arc and neuronal nuclei (NeuN). Bars represent quantitation of Arc immunofluorescence in neurons (expressed as % of Quis treatment) from n = 3 independent experiments; *, p < 0.001 versus Quis. A total of ∼1300 neurons per treatment condition was assessed. Gray bars indicate untreated neurons or neurons treated with indicated antagonists in absence of Quis. B, quantitation of Arc immunofluorescence in neurons (expressed as % of Quis treatment) from n = 3 independent experiments; *, p < 0.001 versus Quis. A total of ∼1100 neurons per treatment condition were assessed. Gray bars indicate untreated control. APV, NMDA antagonist, 100 μ
m
; MK801, NMDA antagonist, 5 μ
m
; CoCl2, nonspecific Ca2+ channel blocker, 1 m
m
; Nifedipine, specific L-type Ca2+ channel blocker, 10 μ
m
. C, shown is a schematic representation of IP3R buffer constructs (18). A plasmid encoding DsRed2 alone, RFP-IP3R-NLS, or NES-RFP-IP3R was transiently transfected into striatal neurons (red) 24 h post-transfection, and neurons were treated with Quis for 1 h, fixed, and stained with Arc (green) and mGluR5 (blue). D, shown is quantitation of Arc immunofluorescence in terms of the number of Arc-positive neurons (expressed as % of DsRed2 alone transfected neurons, left graph) and Arc intensity in transfected neurons (expressed as % of DsRed2 alone transfected neurons, right graph) from n = 3 independent experiments. A total of >85 neurons/plasmid was assessed. *, p < 0.05 compared with DsRed2 alone.
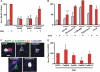
FIGURE 5.
CaMKII and ERK1/2 are involved in intracellular mGluR5-mediated Arc up-regulation. A and B, striatal neurons were treated with Quis (red) in the absence or presence of indicated antagonists (KN62, general CaMK antagonist, 10 μ
m
; KN93, specific CaMKII antagonist, 10 μ
m
; STO609, CaMKK inhibitor, 1.5 μ
m
; U0126, MEK/ERK1/2 antagonist, 1 μ
m
; LY294002, PI3K antagonist, 50 μ
m
; wortmannin, PI3K antagonist, 500 n
m
; rapamycin, mTOR antagonist, 5 n
m
; CysA, cyclosporin A, calcineurin antagonist, 4 μ
m
) for 1 h, fixed, and stained for Arc and neuronal nuclei (NeuN). Bars represent quantitation of Arc immunofluorescence in neurons (expressed as % of Quis treatment) from n = 3–5 independent experiments; *, p < 0.001 _versus_ Quis; #, _p_ < 0.001 _versus_ control. More than 3000 neurons per treatment condition were assessed. _Gray bars_ indicate untreated neurons or neurons treated with indicated antagonists in absence of Quis. _C_, plasmid encoding EGFP alone or EGFP-labeled dominant negative CaMK constructs as indicated were transiently transfected in striatal neurons (_green_), and 24 h post-transfection neurons were treated with Quis for 1 h, fixed, and stained with Arc (_red_) and mGluR5 (_blue_). _D_, shown is quantitation of Arc immunofluorescence in terms of number of Arc-positive neurons (expressed as % of EGFP alone transfected neurons) from _n_ = 5 independent experiments; a total of >250 neurons/plasmid was assessed. *, p < 0.05 compared with EGFP alone.
FIGURE 6.
Intracellular mGluR5-orchestrated Arc protein increase depends on serum response factor. A, mouse striatal neurons prepared from _Srf_f/f (wild type) and _Srf_f/+; NesCre (heterozygote) and _Srf_f/f; NesCre (knockout) animals were stained for SRF immunoreactivity. For simplicity, in the figure cultures are labeled Srf+/+, Srf+/−, and Srf_−/−. B, shown are compiled data from the maximal initial Ca2+ response (Δ_F/Fo, %) from n > 26 Srf+/+, n > 58 Srf+/−, and n > 53 _Srf_−/− neurons, where red bars represent nuclear and blue bar represent cytoplasmic responses. *, p < 0.0001 for all responses compared with basal Ca2+. C, striatal neurons prepared from Srf+/+, Srf+/−, and _Srf_−/− were treated with the indicated drugs for 1 h before staining for Arc (green) and mGluR5 (red). Quis increased Arc expression in mGluR5-positive cell bodies in Srf+/+ and Srf+/− but not in _Srf_−/−. D, shown is quantitation of Arc immunofluorescence in neurons (expressed as the mean ± S.E., compared with control) in the absence (gray) or presence of the indicated ligands (blue for DHPG and red for Quis) treated for 1 h; n = 3. *, p < 0.05 versus control.
FIGURE 7.
Intracellular mGluR5 plays a critical role in glutamate and neuronal activity-induced Arc. A, striatal neurons were pretreated with NMDA antagonist MK801 (5 μ
m
), the AMPA/kainate receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μ
m
), and mGluR1 antagonist CPCCOEt (20 μ
m
) followed by treatment with glutamate (100 μ
m
, 1 h). Glutamate increased Arc expression that was blocked by MPEP but not LY393053. Bars indicate Arc-positive/total neurons compared with control. #, p < 0.05 versus control; n = 4, more than 2500 neurons assessed per treatment. Ctl, control. B, bath application of 15 m
m
KCl-induced Arc in ∼30% striatal neurons. This KCl-regulated induction was decreased ∼28% by MPEP, whereas LY395053 + KCl was not significantly different from KCl alone. Bars indicate Arc positive/total neurons expressed as the percentage of KCl treatment. More than 2000 neurons per treatment from four independent experiments were assessed for Arc immunofluorescence using the High Content Imager. #, p < 0.0001 compared with untreated control. *, p < 0.005 compared with KCl treated alone. C, the proposed model for intracellular mGluR5-mediated transcriptional activation of Arc/Arg3.1 is shown. NPC, nuclear pore complex.
Similar articles
- Intracellular mGluR5 plays a critical role in neuropathic pain.
Vincent K, Cornea VM, Jong YI, Laferrière A, Kumar N, Mickeviciute A, Fung JST, Bandegi P, Ribeiro-da-Silva A, O'Malley KL, Coderre TJ. Vincent K, et al. Nat Commun. 2016 Feb 3;7:10604. doi: 10.1038/ncomms10604. Nat Commun. 2016. PMID: 26837579 Free PMC article. - Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts.
Jong YJ, Kumar V, O'Malley KL. Jong YJ, et al. J Biol Chem. 2009 Dec 18;284(51):35827-38. doi: 10.1074/jbc.M109.046276. J Biol Chem. 2009. PMID: 19840937 Free PMC article. - Ca(2+)/calmodulin-dependent protein kinase II interacts with group I metabotropic glutamate and facilitates receptor endocytosis and ERK1/2 signaling: role of β-amyloid.
Raka F, Di Sebastiano AR, Kulhawy SC, Ribeiro FM, Godin CM, Caetano FA, Angers S, Ferguson SS. Raka F, et al. Mol Brain. 2015 Mar 26;8:21. doi: 10.1186/s13041-015-0111-4. Mol Brain. 2015. PMID: 25885040 Free PMC article. - Roles for Arc in metabotropic glutamate receptor-dependent LTD and synapse elimination: Implications in health and disease.
Wilkerson JR, Albanesi JP, Huber KM. Wilkerson JR, et al. Semin Cell Dev Biol. 2018 May;77:51-62. doi: 10.1016/j.semcdb.2017.09.035. Epub 2017 Oct 14. Semin Cell Dev Biol. 2018. PMID: 28969983 Free PMC article. Review. - Phosphorylation of group I metabotropic glutamate receptors (mGluR1/5) in vitro and in vivo.
Mao LM, Liu XY, Zhang GC, Chu XP, Fibuch EE, Wang LS, Liu Z, Wang JQ. Mao LM, et al. Neuropharmacology. 2008 Sep;55(4):403-8. doi: 10.1016/j.neuropharm.2008.05.034. Epub 2008 Jun 10. Neuropharmacology. 2008. PMID: 18585398 Free PMC article. Review.
Cited by
- Characterization of a mGluR5 Knockout Rat Model with Hallmarks of Fragile X Syndrome.
Dahl V, Helmbrecht H, Rios Sigler A, Hildahl K, Sullivan H, Janakiraman S, Jasti S, Nance E. Dahl V, et al. Life (Basel). 2022 Aug 25;12(9):1308. doi: 10.3390/life12091308. Life (Basel). 2022. PMID: 36143345 Free PMC article. - Intracellular mGluR5 plays a critical role in neuropathic pain.
Vincent K, Cornea VM, Jong YI, Laferrière A, Kumar N, Mickeviciute A, Fung JST, Bandegi P, Ribeiro-da-Silva A, O'Malley KL, Coderre TJ. Vincent K, et al. Nat Commun. 2016 Feb 3;7:10604. doi: 10.1038/ncomms10604. Nat Commun. 2016. PMID: 26837579 Free PMC article. - Functional G protein-coupled receptors on nuclei from brain and primary cultured neurons.
Jong YJ, O'Malley KL. Jong YJ, et al. Methods Mol Biol. 2015;1234:113-21. doi: 10.1007/978-1-4939-1755-6_10. Methods Mol Biol. 2015. PMID: 25304352 Free PMC article. - Updates on the Physiopathology of Group I Metabotropic Glutamate Receptors (mGluRI)-Dependent Long-Term Depression.
Mango D, Ledonne A. Mango D, et al. Cells. 2023 Jun 8;12(12):1588. doi: 10.3390/cells12121588. Cells. 2023. PMID: 37371058 Free PMC article. Review. - Chaperoning G protein-coupled receptors: from cell biology to therapeutics.
Tao YX, Conn PM. Tao YX, et al. Endocr Rev. 2014 Aug;35(4):602-47. doi: 10.1210/er.2013-1121. Epub 2014 Mar 24. Endocr Rev. 2014. PMID: 24661201 Free PMC article. Review.
References
- Catania M. V., D'Antoni S., Bonaccorso C. M., Aronica E., Bear M. F., Nicoletti F. (2007) Group I metabotropic glutamate receptors. A role in neurodevelopmental disorders? Mol. Neurobiol. 35, 298–307 - PubMed
- Gasparini F., Bilbe G., Gomez-Mancilla B., Spooren W. (2008) mGluR5 antagonists. Discovery, characterization, and drug development. Curr. Opin. Drug Discov. Devel. 11, 655–665 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS057105/NS/NINDS NIH HHS/United States
- MH57817/MH/NIMH NIH HHS/United States
- R21 MH069646/MH/NIMH NIH HHS/United States
- R01 MH057817/MH/NIMH NIH HHS/United States
- P30 NS057105/NS/NINDS NIH HHS/United States
- MH69646/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous