Phosphatidylinositol 4,5-bisphosphate (PIP(2)) controls magnesium gatekeeper TRPM6 activity - PubMed (original) (raw)
Phosphatidylinositol 4,5-bisphosphate (PIP(2)) controls magnesium gatekeeper TRPM6 activity
Jia Xie et al. Sci Rep. 2011.
Erratum in
- Sci Rep. 2012;2:559
Abstract
TRPM6 is crucial for human Mg2+ homeostasis as patients carrying TRPM6 mutations develop hypomagnesemia and secondary hypocalcemia (HSH). However, the activation mechanism of TRPM6 has remained unknown. Here we demonstrate that phosphatidylinositol-4,5-bisphophate (PIP2) controls TRPM6 activation and Mg2+ influx. Stimulation of PLC-coupled M1-receptors to deplete PIP2 potently inactivates TRPM6. Translocation of over-expressed 5-phosphatase to cell membrane to specifically hydrolyze PIP2 also completely inhibits TRPM6. Moreover, depolarization-induced-activation of the voltage-sensitive-phosphatase (Ci-VSP) simultaneously depletes PIP2 and inhibits TRPM6. PLC-activation induced PIP2-depletion not only inhibits TRPM6, but also abolishes TRPM6-mediated Mg2+ influx.Furthermore, neutralization of basic residues in the TRP domain leads to nonfunctional or dysfunctional mutants with reduced activity by PIP2, suggesting that they are likely to participate in interactions with PIP2.Our data indicate that PIP2 is required for TRPM6 channel function; hydrolysis of PIP2 by PLC-coupled hormones/agonists may constitute an important pathway for TRPM6 gating, and perhaps Mg2+ homeostasis.
Figures
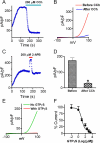
Figure 1. TRPM6 current is inhibited by CCh stimulation of the M1 receptor.
(A) A representative recording in HM1 cells transfected with TRPM6. Currents were elicited by 100 ms voltage ramps ranging from −100 to +100 mV. Application of 200 μM CCh to activate M1 receptor completely inhibited TRPM6 current. (B) Time-dependent changes of outward TRPM6 current measured at +100 mV before and after 200 μM CCh application. (C) Potentiation of the currents by 2-APB was used to confirm that the recordings were TRPM6 currents. (D) Mean current densities of TRPM6 before and after CCh (n = 10, * p<0.05). (E) Representative traces of TRPM6 currents recorded from different cells using pipette solution with or without 1 mM GTPγS. (F) Concentration-dependent effects of GTPγS on TRPM6. Currents recorded at various GTPγS concentrations were normalized to the current recorded without GTPγS in the pipette solution. Best fit of the dose-response curve yielded IC50 = 10.1 μM (n = 5–9 cells for each GTPγS concentration).
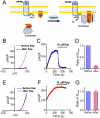
Figure 2. Membrane tethering of type IV 5-ptase reduces membrane PIP2 and suppresses TRPM6 current.
(A) Schematic diagram demonstrating how the rapamycin (Rap) inducible PIP2 specific phosphatase system works. (B) Representative recordings of TRPM6 before and after Rap application in cells transfected with GFP-TRPM6, FKBP-Inp54p and Lyn-FRB. (C) Time course of TRPM6 inhibition by perfusing 10 μM Rap. (D) Average inhibition of TRPM6 by 10 μM Rap (n = 12) in cells transfected with TRPM6, FKBP-Inp54p and Lyn-FRB. (E) Representative recordings of TRPM6 before and after Rap application in cells transfected with TRPM6 alone. (F) Time-dependent changes of TRPM6 before and after 10 μM Rap in cells shown in (E). (G) Mean current densities of TRPM6 before and after Rap. Rap did not produce inhibition on TRPM6 in cells transfected with TRPM6 alone.
Figure 3. Simultaneous monitoring of PIP2 depletion and TRPM6 inactivation.
(A) The protocol for activation of Ci-VSP and recording of TRPM6 currents. Cells were held at −60 mV to allow dialysis of pipette solution without activation of Ci-VSP. Voltage Ramp ranging from −120 to +100 mV was applied to activate Ci-VSP and record TRPM6 currents. (B–C) Representative traces recorded before and after activation of Ci-VSP in cells transfected with TRPM6 and Ci-VSP (B) and TRPM6 alone (C). (D) Time-dependent changes of TRPM6 outward current measured at +100 mV in cells transfected with TRPM6+Ci-VSP and TRPM6 alone. (E–F) Normalized currents before and after depolarization from cells with TRPM6+Ci-VSP transfection (E) and with TRPM6 transfection alone (F) (n = 8 for each group). (G) Representative GFP-PH domain sub-cellular location at different time points upon depolarization. Cells were co-transfected with GFP-PH, Ci-VSP and TRPM6. (H) Kinetics of cytosol fluorescence increment and TRPM6 current inhibition. The intensity of fluorescence and current amplitude of TRPM6 at each time-point were normalized to the initial values, respectively. The time constants obtained by mono-exponential fit were 24.2± 2.5 s for PH-GFP translocation, and 36.5±6.9 s for TRPM6 inactivation.
Figure 4. PIP2 binding sites of TRPM6.
(A) Alignment of the TRP domain of TRPM6, TRPM7 and TRPM8. The highlighted residues in TRPM8 are the PIP2 binding sites. (B) Representative traces of WT-TRPM6 and mutants K1085Q, R1088Q, and K1098Q. (C–D) Effects of 2-APB on the mutants K1085Q and R1088Q. (E) Normalized mean current density of TRPM6 mutants in comparison with WT TRPM6 (n = 10 *p<0.05; ** P<0.01). Mutants K1098Q and the double mutants K1085Q/R1088Q did not produce any current. (F) Average changes of current amplitude by 2-APB (200 μM). 2-APB did not have any effect on the non-functional mutants K1098Q and K1085Q/R1088Q.
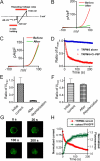
Figure 5. Plasma membrane expression of WT TRPM6 and the putative PIP2 binding-site mutants.
(A) Western-blot (with GFP antibody) detection of proteins of the WT and mutants in the plasma membrane portion and in total lysate. GADPH was used as a negative control for the plasma membrane protein. (B) Mean relative expression level of WT TRPM6 and the mutants in the plasma membrane versus total protein. No statistically significant difference was observed (n = 3).
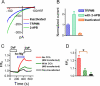
Figure 6. Effects of PIP2 on channel activities of TRPM6 and its mutant in the inside-out patches.
(A) Representative recordings of WT TRPM6 single channel currents in an inside-out patch at -50 mV recorded in divalent free solution (DVF). PIP2 (DiC8-PIP2) at 10 μM was applied to the patch after completely rundown of channel opening. (B) Single channel currents of R1088Q recorded at −50 mV right after formation of excised patch, after rundown, and upon application of 10 μM PIP2. (C) Average open probability of TRPM6 (n = 8) and R1088Q (n = 7) before and after completely rundown. (D) Percentage of channel open probabilities rescued by 10 μM PIP2 after rundown of TRPM6 and R1088Q. The effect of DiC8-PIP2 was reversible and reproducible in the same patches.
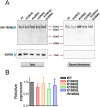
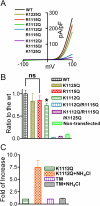
Figure 7. Mutation of the putative PIP2 binding residues of TRPM7 disrupts channel function.
(A) Representative traces of WT TRPM7 and its mutants. The double mutant K1112Q/R1115Q (DM) and the triple mutant K1112Q/R1115Q/K1125Q did not produce currents. (B) Mean current densities of the WT and its mutants (n = 8–13, *p<0.05). (C) Effects of NH4Cl on K1112Q, and triple mutant K1112Q/R1115Q/K1125Q (TM) were tested by using pipette solution containing 1 mM free Mg2+. Perfusion of 30 mM NH4Cl can increase intracellular pH thereby releasing PIP2 sequestered by Mg2+, thus, the effects of PIP2 on the channel activity is manifested. NH4Cl significantly increase the current amplitude of K1112Q, but failed to induce any change in the DM and TM transfected cells, indicating that the double mutant K1112Q/R1115Q and the triple mutant K1112Q/R1115Q/K1125Q have no ability to sense the changes of PIP2 levels.
Figure 8. Depletion of PIP2 inhibits Mg2+ currents and eliminates Mg2+ influx through TRPM6.
(A) Mg2+ currents recorded using isotonic extracellular Mg2+ in cells transfected with either TRPM6 alone (blue) or TRPM6+Ci-VSP (red). Mg2+ current (blue) was significantly increased by 2-APB (green), and completely inhibited after PIP2 depletion induced by activation of Ci-VSP. (B) Normalized Mg2+ current amplitude (n = 9). (C) Mg2+ influx through TRPM6 under control conditions and after PIP2 hydrolysis by 200 μM CCh. Changes of Mg2+ influx were measured in isotonic Mg2+ extracellular solution. Note the normalized fluorescence intensity (F/F0) induced by isotonic Mg2+ was largely diminished by CCH. (D) Average changes of F/F0 (n = 33∼36 in each group).
Similar articles
- The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate.
Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. Nilius B, et al. EMBO J. 2006 Feb 8;25(3):467-78. doi: 10.1038/sj.emboj.7600963. Epub 2006 Jan 19. EMBO J. 2006. PMID: 16424899 Free PMC article. - TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption.
Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. Voets T, et al. J Biol Chem. 2004 Jan 2;279(1):19-25. doi: 10.1074/jbc.M311201200. Epub 2003 Oct 23. J Biol Chem. 2004. PMID: 14576148 - Charge Shielding of PIP2 by Cations Regulates Enzyme Activity of Phospholipase C.
Seo JB, Jung SR, Huang W, Zhang Q, Koh DS. Seo JB, et al. PLoS One. 2015 Dec 11;10(12):e0144432. doi: 10.1371/journal.pone.0144432. eCollection 2015. PLoS One. 2015. PMID: 26658739 Free PMC article. - TRPM6.
Chubanov V, Gudermann T. Chubanov V, et al. Handb Exp Pharmacol. 2014;222:503-20. doi: 10.1007/978-3-642-54215-2_20. Handb Exp Pharmacol. 2014. PMID: 24756719 Review. - Epithelial Mg2+ channel TRPM6: insight into the molecular regulation.
van der Wijst J, Hoenderop JG, Bindels RJ. van der Wijst J, et al. Magnes Res. 2009 Sep;22(3):127-32. Magnes Res. 2009. PMID: 19780399 Review.
Cited by
- Role of TRP channels in the cardiovascular system.
Yue Z, Xie J, Yu AS, Stock J, Du J, Yue L. Yue Z, et al. Am J Physiol Heart Circ Physiol. 2015 Feb 1;308(3):H157-82. doi: 10.1152/ajpheart.00457.2014. Epub 2014 Nov 21. Am J Physiol Heart Circ Physiol. 2015. PMID: 25416190 Free PMC article. Review. - Natural and Synthetic Modulators of the TRPM7 Channel.
Chubanov V, Schäfer S, Ferioli S, Gudermann T. Chubanov V, et al. Cells. 2014 Nov 27;3(4):1089-101. doi: 10.3390/cells3041089. Cells. 2014. PMID: 25437439 Free PMC article. Review. - The region adjacent to the C-end of the inner gate in transient receptor potential melastatin 8 (TRPM8) channels plays a central role in allosteric channel activation.
Taberner FJ, López-Córdoba A, Fernández-Ballester G, Korchev Y, Ferrer-Montiel A. Taberner FJ, et al. J Biol Chem. 2014 Oct 10;289(41):28579-94. doi: 10.1074/jbc.M114.577478. Epub 2014 Aug 25. J Biol Chem. 2014. PMID: 25157108 Free PMC article. - Lipid agonism: The PIP2 paradigm of ligand-gated ion channels.
Hansen SB. Hansen SB. Biochim Biophys Acta. 2015 May;1851(5):620-8. doi: 10.1016/j.bbalip.2015.01.011. Epub 2015 Jan 26. Biochim Biophys Acta. 2015. PMID: 25633344 Free PMC article. Review. - Phosphoinositide isoforms determine compartment-specific ion channel activity.
Zhang X, Li X, Xu H. Zhang X, et al. Proc Natl Acad Sci U S A. 2012 Jul 10;109(28):11384-9. doi: 10.1073/pnas.1202194109. Epub 2012 Jun 25. Proc Natl Acad Sci U S A. 2012. PMID: 22733759 Free PMC article.
References
- Quamme G. A. Molecular identification of ancient and modern mammalian magnesium transporters. Am J Physiol Cell Physiol 298, C407–429 (2010). - PubMed
- Touyz R. M. Magnesium in clinical medicine. Front Biosci 9, 1278–1293 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM080753/GM/NIGMS NIH HHS/United States
- R01 HL078960/HL/NHLBI NIH HHS/United States
- 2R01HL078960/HL/NHLBI NIH HHS/United States
- 1R01GM080753/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous