A novel platform for detection of CK+ and CK- CTCs - PubMed (original) (raw)
doi: 10.1158/2159-8290.CD-11-0215. Epub 2011 Nov 3.
Farideh Z Bischoff, Julie Ann Mayer, Karina L Wong, Tam Pham, Justin Bottsford-Miller, Rebecca L Stone, Yvonne G Lin, Padmavathi Jaladurgam, Ju Won Roh, Blake W Goodman, William M Merritt, Tony J Pircher, Stephen D Mikolajczyk, Alpa M Nick, Joseph Celestino, Cathy Eng, Lee M Ellis, Michael T Deavers, Anil K Sood
Affiliations
- PMID: 22180853
- PMCID: PMC3237635
- DOI: 10.1158/2159-8290.CD-11-0215
A novel platform for detection of CK+ and CK- CTCs
Chad V Pecot et al. Cancer Discov. 2011 Dec.
Abstract
Metastasis is a complex, multistep process that begins with the epithelial-mesenchymal transition (EMT). Circulating tumor cells (CTC) are believed to have undergone EMT and thus lack or express low levels of epithelial markers commonly used for enrichment and/or detection of such cells. However, most current CTC detection methods target only EpCAM and/or cytokeratin (CK) to enrich epithelial CTCs, resulting in failure to recognize other, perhaps more important, CTC phenotypes that lack expression of these markers. Here, we describe a population of complex aneuploid CTCs that do not express CK or CD45 antigen in patients with breast, ovarian, or colorectal cancer. These cells were not observed in healthy subjects. We show that the primary epithelial tumors were characterized by similar complex aneuploidy, indicating conversion to an EMT phenotype in the captured cells. Collectively, our study provides a new method for highly efficient capture of previously unrecognized populations of CTCs.
Significance: Current assays for CTC capture likely miss populations of cells that have undergone EMT. Capture and study of CTCs that have undergone EMT would allow a better understanding of the mechanisms driving metastasis.
Conflict of interest statement
Conflict of interest statement: The authors F.Z.B., T.J.P., S.D.M., J.A.M., K.W., and T.P. are employees of Biocept Incorporated.
Figures
Figure 1
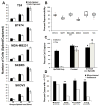
Efficiency and reproducibility of cell capture and comparison to CellSearch® technology. (a) Median number of captured carcinoma cells after ex vivo spiking of approximately 10, 25, or 50 cells into 10 mL human blood when using the antibody cocktail against cells of varying EpCAM expression. Each spike was performed in triplicate. (b) Percent reproducibility of cell capture after 10 separate ex vivo spikes into blood (pink-minimum outlier, red-maximum outlier). (c) Percentages of captured SKOV3 and T24 carcinoma cells after ex vivo spiking of approximately 150 cells into 10 mL human blood when using EpCAM antibody only, the antibody cocktail, or the antibody cocktail without the EpCAM antibody. (d) Comparison with the CellSearch® platform for CK+ CTCs captured from patients with breast, colorectal, lung, or prostate carcinomas. a: Tumor types approved for CTC enumeration using CellSearch®, b: For samples from breast, colorectal and lung cancer patients, the antibody cocktail (AC15) composed of 10 monoclonal antibodies was used, c: For samples from prostate cancer patients, the antibody cocktail (AC16) composed of 11 monoclonal antibodies was used. *P < 0.05, ** P = 0.001, ‡ P = 0.0001.
Figure 2
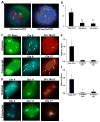
Capture of CK+ and CK− complex aneuploid CTCs in breast, ovarian or colorectal cancers. (a) Representative images illustrating detection of HER2+/CK+ and HER2+/CK− CTCs. Both cells display a >2.2 HER2/Centromere 17 ratio, confirming positive HER2 amplification. (b) Comparison of total CK+/CD45−, CK+/CD45−/HER2+ and CK−/CD45−/HER2+ cells from advanced-stage breast cancer patients. (c) Capture of circulating ovarian (top) and colorectal (bottom) carcinoma cells that stain for cytokeratin. Subsequent FISH shows an ovarian cancer cell with trisomy in chromosome 8 (blue) and monosomy in region 20q12 (red), whereas the colorectal cancer cell has trisomy in chromosome 8 and tetrasomy in chromosome 17 (orange, arrows). (d) Capture of CK-negative circulating ovarian (top) and colorectal (bottom) carcinoma cells. FISH of an ovarian cancer cell with trisomy in chromosome 8 (blue), monosomy in chromosome 11 (green), and tetrasomy in region 20q12 (orange), whereas the colorectal cancer cell has trisomy in chromosomes 8 (blue) and 11 (green) and monosomy in region 20q12. The average number of total cytokeratin, complex aneuploid cytokeratin-positive, and cytokeratin-negative circulating tumor cells per milliliter of blood is shown for (e) ovarian and (f) colorectal cancer patients. *P < 0.05, **P = 0.007.
Figure 3
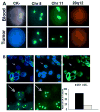
Matched CK-positive and negative cells in circulation and primary tumor. (a) CK-negative ovarian cancer cells identified in circulation (top) at the time of surgical resection have similar aneuploidy as regions in the tumor (bottom). Represented are cells with trisomy of chromosome 8. (b) CK staining of ovarian carcinoma samples reveals CK-negative cells with aneuploidy (arrows) similar to those detected in circulation. Approximately 20% of the tumor had such CK-negative cells.
Figure 4
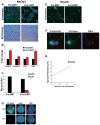
Characterization and capture of cytokeratin-negative cells after induction of EMT. (a) SKOV3 cells were either grown in regular culture media (Pre-EMT) or in serum-free media with 10 ng/mL TGF-β (Post-EMT) for 72 hours. Pictured are representative immunofluorescent images (top) of Pre-EMT cells demonstrating 100% CK expression and areas of Post-EMT cells with absent CK expression. Approximately 20% of post-EMT cells were found to have complete loss of cytokeratin expression. Phase contrast images of the same cells (bottom) demonstrate a morphologic change characteristic of EMT. (b) Quantitative real-time PCR for markers of EMT of SKOV3 cells with and without TGF-β treatment for 72 hours. (c) Following 72 hours, pre- and post-EMT cells were spiked ex vivo into mouse blood and run through the CEE™ microchannel. All pre-EMT cells that were captured were CK+ and had complex aneuploidy, while 16% of post-EMT cells were CK− and had similar complex aneuploidy. The bar graph represents ratios of CK+ and CK− complex aneuploid captured cells in each group. (d) Representative images of CK+ and CK− complex aneuploid SKOV3 cells are shown from within the microchannel. (e) HeyA8 cells were cultured in regular media (Pre-EMT) or serum-free media with 10 ng/mL TGF-β(Post-EMT) for 72 hours. Representative immunofluorescent images of Pre-EMT cells demonstrating nearly 100% CK expression and TGF-β treated cells with absent CK expression are shown. Approximately 60% of TGF-β treated cells were found to have complete loss of cytokeratin expression. (f) HeyA8 cells were injected into 10 mice to establish a metastatic ovarian model. Once moribund, blood was collected from each mouse by cardiac puncture. Pictured are a CK+ and CK− CTC within the microchannel demonstrating hyperploidy of chromosomes 11 and 17. (g) Correlation of total aggregate tumor burden with enumeration of complex aneuploid CK− CTCs by mouse.
Similar articles
- Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition.
Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, Krahn T, von Ahsen O. Gorges TM, et al. BMC Cancer. 2012 May 16;12:178. doi: 10.1186/1471-2407-12-178. BMC Cancer. 2012. PMID: 22591372 Free PMC article. - Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients.
Deng G, Herrler M, Burgess D, Manna E, Krag D, Burke JF. Deng G, et al. Breast Cancer Res. 2008;10(4):R69. doi: 10.1186/bcr2131. Epub 2008 Aug 7. Breast Cancer Res. 2008. PMID: 18687126 Free PMC article. - EpCAM-independent isolation of circulating tumor cells with epithelial-to-mesenchymal transition and cancer stem cell phenotypes using ApoStream® in patients with breast cancer treated with primary systemic therapy.
Le Du F, Fujii T, Kida K, Davis DW, Park M, Liu DD, Wu W, Chavez-MacGregor M, Barcenas CH, Valero V, Tripathy D, Reuben JM, Ueno NT. Le Du F, et al. PLoS One. 2020 Mar 26;15(3):e0229903. doi: 10.1371/journal.pone.0229903. eCollection 2020. PLoS One. 2020. PMID: 32214335 Free PMC article. - Current and future role of circulating tumor cells in patients with epithelial ovarian cancer.
Van Berckelaer C, Brouwers AJ, Peeters DJ, Tjalma W, Trinh XB, van Dam PA. Van Berckelaer C, et al. Eur J Surg Oncol. 2016 Dec;42(12):1772-1779. doi: 10.1016/j.ejso.2016.05.010. Epub 2016 May 25. Eur J Surg Oncol. 2016. PMID: 27265041 Review. - Circulating Tumor Cells and Implications of the Epithelial-to-Mesenchymal Transition.
Lowes LE, Allan AL. Lowes LE, et al. Adv Clin Chem. 2018;83:121-181. doi: 10.1016/bs.acc.2017.10.004. Epub 2017 Dec 21. Adv Clin Chem. 2018. PMID: 29304900 Review.
Cited by
- Hematogenous metastasis of ovarian cancer: rethinking mode of spread.
Pradeep S, Kim SW, Wu SY, Nishimura M, Chaluvally-Raghavan P, Miyake T, Pecot CV, Kim SJ, Choi HJ, Bischoff FZ, Mayer JA, Huang L, Nick AM, Hall CS, Rodriguez-Aguayo C, Zand B, Dalton HJ, Arumugam T, Lee HJ, Han HD, Cho MS, Rupaimoole R, Mangala LS, Sehgal V, Oh SC, Liu J, Lee JS, Coleman RL, Ram P, Lopez-Berestein G, Fidler IJ, Sood AK. Pradeep S, et al. Cancer Cell. 2014 Jul 14;26(1):77-91. doi: 10.1016/j.ccr.2014.05.002. Cancer Cell. 2014. PMID: 25026212 Free PMC article. - High-throughput screening of rare metabolically active tumor cells in pleural effusion and peripheral blood of lung cancer patients.
Tang Y, Wang Z, Li Z, Kim J, Deng Y, Li Y, Heath JR, Wei W, Lu S, Shi Q. Tang Y, et al. Proc Natl Acad Sci U S A. 2017 Mar 7;114(10):2544-2549. doi: 10.1073/pnas.1612229114. Epub 2017 Feb 21. Proc Natl Acad Sci U S A. 2017. PMID: 28223509 Free PMC article. - Circulating tumor cell enrichment based on physical properties.
Harouaka RA, Nisic M, Zheng SY. Harouaka RA, et al. J Lab Autom. 2013 Dec;18(6):455-68. doi: 10.1177/2211068213494391. Epub 2013 Jul 5. J Lab Autom. 2013. PMID: 23832928 Free PMC article. Review. - Epithelial mesenchymal transition: a new insight into the detection of circulating tumor cells.
Barrière G, Tartary M, Rigaud M. Barrière G, et al. ISRN Oncol. 2012;2012:382010. doi: 10.5402/2012/382010. Epub 2012 Apr 11. ISRN Oncol. 2012. PMID: 22577580 Free PMC article. - Single-Cell, Multiplexed Protein Detection of Rare Tumor Cells Based on a Beads-on-Barcode Antibody Microarray.
Yang L, Wang Z, Deng Y, Li Y, Wei W, Shi Q. Yang L, et al. Anal Chem. 2016 Nov 15;88(22):11077-11083. doi: 10.1021/acs.analchem.6b03086. Epub 2016 Sep 28. Anal Chem. 2016. PMID: 27644430 Free PMC article.
References
- Thiery JP. Epithelial-Mesenchymal Transitions in Tumour Progression. Nature Reviews Cancer. 2002;2:442–54. - PubMed
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clinical Cancer Research. 2004;10:6897–904. - PubMed
- Coumans FAW, Doggen CJM, Attard G, de Bono JS, Terstappen LWWM. All circulating EpCAM+CK+CD45− objects predict overall survival in castration-resistant prostate cancer. Annals of Oncology. 2010:1–7. - PubMed
- Goodman OB, Jr, Fink LM, Symanowski JT, Wong B, Grobaski B, Pomerantz D, et al. Circulating Tumor Cells in Patients with Castration-Resistant Prostate Cancer Baseline Values and Correlation with Prognostic Factors. Cancer Epidemiology Biomarker Prevention. 2009;18:1904–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- CA016672/CA/NCI NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- CA110793/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- R01 CA128797/CA/NCI NIH HHS/United States
- T32 CA009666/CA/NCI NIH HHS/United States
- T32 CA101642/CA/NCI NIH HHS/United States
- CA109298/CA/NCI NIH HHS/United States
- CA128797/CA/NCI NIH HHS/United States
- R01 CA109298/CA/NCI NIH HHS/United States
- RC2GM092599/GM/NIGMS NIH HHS/United States
- U54 CA151668/CA/NCI NIH HHS/United States
- R01 CA110793/CA/NCI NIH HHS/United States
- RC2 GM092599/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous