Nanodomain coupling between Ca²⁺ channels and sensors of exocytosis at fast mammalian synapses - PubMed (original) (raw)
Review
Nanodomain coupling between Ca²⁺ channels and sensors of exocytosis at fast mammalian synapses
Emmanuel Eggermann et al. Nat Rev Neurosci. 2011.
Abstract
The physical distance between presynaptic Ca(2+) channels and the Ca(2+) sensors that trigger exocytosis of neurotransmitter-containing vesicles is a key determinant of the signalling properties of synapses in the nervous system. Recent functional analysis indicates that in some fast central synapses, transmitter release is triggered by a small number of Ca(2+) channels that are coupled to Ca(2+) sensors at the nanometre scale. Molecular analysis suggests that this tight coupling is generated by protein-protein interactions involving Ca(2+) channels, Ca(2+) sensors and various other synaptic proteins. Nanodomain coupling has several functional advantages, as it increases the efficacy, speed and energy efficiency of synaptic transmission.
Figures
Figure 1. Model synapses used for the analysis of Ca2+ channel–sensor coupling
a | The frog neuromuscular junction, which is a classical preparation for the analysis of synaptic transmission. This synapse is formed between motor axons (yellow) and skeletal muscle fibres (pink). A technical advantage is the 1:1 innervation (1 motor axon:1 muscle fibre). Furthermore, the structure of this synapse has been studied extensively. Presynaptic access, however, is not possible. b | The squid giant synapse. This synapse is established between second and third order giant nerve fibres in the stellate ganglion of the squid. A technical advantage is that presynaptic elements can be recorded directly with sharp microelectrodes. c | The calyx of Held in the auditory brainstem,. This synapse is formed between the globular bushy cells in the cochlear nucleus and the neurons of the medial nucleus of the trapezoid body (MNTB). A technical advantage of this synapse is that presynaptic terminals can be recorded directly with patch-clamp techniques. However, a disadvantage is that recordings from older animals (>postnatal day 8–10) are difficult. d | The hippocampal dentate gyrus basket cell synapse. This synapse is established between fast-spiking, parvalbumin-expressing basket cells in the hippocampus (yellow) and postsynaptic target cells (in this case granule cells, blue). e | The cerebellar basket cell synapse. This synapse is established between parvalbumin-expressing basket cells in the cerebellum (yellow) and postsynaptic target cells (in this case Purkinje cells, blue). In hippocampal and cerebellar basket cell synapses, paired recordings between presynaptic and postsynaptic neurons can be obtained with high success rates because of the relatively high connectivity. A disadvantage of these synapses is that presynaptic terminals cannot be routinely recorded. Part a is modified, with permission, from REF. © (1992) Sinauer. Part b is modified, with permission, from REF. © (1957) The Rockefeller University Press. Part c is modified, with permission, from REF. © (2002) Macmillan Publishers Ltd. All rights reserved.
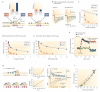
Figure 2. Experimental determination of the coupling distance and the number of open Ca2+ channels that mediate transmitter release
a | Ca2+ chelators with different on rates are used to probe the distance between Ca2+ channels and sensors. In a tight coupling regime (left), only the fast Ca2+ chelator BAPTA, but not the slow Ca2+ chelator EGTA, will capture the Ca2+ on its way from the source to the sensor. By contrast, in a loose coupling regime (right), both chelators will be effective, according to their affinity values, which are comparable. b | Effects of 30 mM EGTA on unitary inhibitory postsynaptic currents (IPSCs) at the hippocampal basket cell–granule cell synapse under steady-state conditions. Orange traces, presynaptic action potentials; black traces, IPSCs; green traces, averages. Note that EGTA has only minimal effects at this synapse. c | Concentration dependence of the effects of BAPTA and EGTA at the hippocampal basket cell–granule cell synapse. Lines represent predictions of a reaction–diffusion model simplified by linearization (continuous lines, predictions for a single Ca2+ channel; dashed lines, predictions for a cluster of multiple Ca2+ channels). The best description of the experimental data was obtained assuming a coupling distance of 12 nm. d,e | Target-cell-specific differences in the coupling distance. Concentration dependence of the effects of BAPTA and EGTA at glutamatergic synapses formed by pyramidal neurons in somatosensory cortex on bitufted interneurons (presumably representing somatostatin-positive subtypes) and multipolar interneurons (presumably representing parvalbumin-expressing subtypes). In the pyramidal neuron–multipolar interneuron synapses, synaptic transmission is only weakly sensitive to EGTA, suggesting tight coupling between Ca2+ channels and sensors. f | Presynaptic plasticity changes the contribution of N-type Ca2+ channels to transmitter release at glutamatergic perforant path synapses on hippocampal CA1 pyramidal neurons. After 200 Hz tetanic stimulation (arrow, Tet), inducing a presumably presynaptic form of long-term potentiation, the amount of block by ω-conotoxin GVIa (ω-Ctx GVIa), a selective N-type channel blocker, increases, suggesting that transmission becomes increasingly dependent on N-type channels. g | A slow calcium channel blocker can be used to estimate the number of open channels required for neurotransmission. In a multiple channel coupling scenario (upper panel), blocking Ca2+ channels with a slow blocker scales the Ca2+ transient at the vesicular Ca2+ sensor, reducing transmitter release supralinearly. In a single-channel scenario (lower panel), blocking Ca2+ channels sequentially eliminates channel–vesicle nanocomplexes, inhibiting transmitter release linearly. h | Ca2+ transients (upper traces) and IPSCs (lower traces) at the hippocampal basket cell–granule cell synapse before and after application of ω-agatoxin IVa (ω-Aga IVa). Corresponding scale bars are at the bottom. Note that the toxin reduces Ca2+ transients and IPSCs to a comparable extent. Presynaptic Ca2+ transients were measured as relative fluorescence changes (Δ_F_/F_0) using the Ca2+ indicator dye Oregon Green BAPTA1. i | Plot of peak amplitudes of synaptic currents as a measure of exocytosis against Δ_F/_F_0 as a measure of Ca2+ inflow (both normalized to the respective control value). The blue curves show the predictions of a binomial model of Ca2+ channel block with different numbers of open Ca2+ channels (n = 1, 2 or 10). The red curve shows free fit with a power function. Note that the best fit of the experimental observations can be obtained with a model assuming two or three Ca2+ channels. Parts b and c are reproduced, with permission, from REF. © (2008) Elsevier. Parts d and e are reproduced, with permission, from REF. © (2001) Wiley-Blackwell. Part f is reproduced, with permission, from REF. © (2009) Elsevier. Parts h and i are reproduced, with permission, from REF. © (2010) Macmillan Publishers Ltd. All rights reserved. EPSP, excitatory postsynaptic potential; fEPSP, field EPSP.
Figure 3. Molecular mechanisms of nanodomain coupling
a | Space filling models of protein complexes in the active zone. A synaptic vesicle is surrounded by several proteins. Only a single copy of each protein is depicted. b illustration of the proposed function of RAB3-interacting molecule (RIM) as a tether in the active zone. Both RIM and RIM binding protein (RIM-BP) bind to the C terminus (C) of the Ca2+ channel. Furthermore, the N terminus (N) of RIM binds to RAB3A. As RAB3A is a vesicular protein, the complex links Ca2+ channels to synaptic vesicles. c | Genetic elimination of RIMs changes the dependency of inhibitory postsynaptic current (IPSC) amplitudes on extracellular Ca2+ concentration at GABAergic synapses. Left, dose–effect curves in control synapses, RIM conditional double knockout (cDKO) synapses, and after rescue with recombinantly expressed RIM1α (cDKO + RIM1α). Right, summary bar graph of EC50 (the concentration of an agonist at which 50% of the response is seen) values in the three conditions. d | Genetic elimination of RIMs changes the coupling between Ca2+ source and Ca2+ sensor at GABAergic synapses. Left, IPSCs in control synapses (top) and in RIM double knockout synapses (bottom) at different times during application of EGTA acetoxymethyl ester (EGTA-AM). Centre, time course of inhibitory effects of EGTA-AM at control synapses (green) and double knockout synapses (blue). Right, time constants of the onset of the effects of EGTA-AM. EGTA-AM acts more rapidly in the RIM double knockout mouse, suggesting a looser coupling between Ca2+ channels and sensors of exocytosis. Although the experiments were performed at cultured hippocampal inhibitory synapses, it is likely that at least a subset includes output synapses from parvalbumin-expressing, fast-spiking interneurons. Image in a is reproduced, with permission, from REF. © (2010) PNAS. Part b is modified, with permission, from REF. © (2011) Elsevier. Parts c and d are reproduced, with permission, from REF. © (2011) Elsevier. BKCa, large conductance calcium-activated potassium channel; CaMKII, calcium/calmodulin-dependent protein kinase type II; Cav, voltage-gated calcium channel; KCTD16, K+ channel tetramerization domain-containing protein 16; PMCA, plasma membrane Ca2+ ATPase; PP2B, protein phosphatase 2B; Syt, synaptotagmin; RAB3A, small G protein localized on synaptic vesicles; rPTP, receptor protein tyrosine phosphatase.
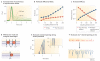
Figure 4. Functional consequences of nanodomain coupling
a | Tight coupling increases the ratio of synchronous to asynchronous release by increasing the ratio of peak Ca2+ to residual Ca2+. Traces show normalized action potential-evoked Ca2+ transients at distances between 20 nm and 200 nm (step size 20 nm). The fast component of the Ca2+ transient will drive synchronous release, whereas the slow component will initiate asynchronous release. The red dashed line represents the presynaptic action potential. b | Tight coupling reduces the component of the synaptic delay that is caused by diffusion of Ca2+ (red circles and curve) and, in parallel, increases the temporal precision of release in relation to the presynaptic action potential (‘half duration’; blue circles and curve). c | Tight coupling increases release probability and thus synaptic efficacy (red circles and curve) and, in relative terms, decreases asynchronous release (blue circles and curve). d | Tight coupling reduces the presynaptic Ca2+ load and thus introduces energetic advantages. Na+/K+-ATPase, Na+/Ca2+ exchanger and Ca2+-ATPase are depicted schematically. Na+/Ca2+ exchanger and Ca2+-ATPase are the main Ca2+ extrusion mechanisms in the presynaptic plasma membrane. The Ca2+-ATPase is primary active, that is, directly dependent on the hydrolysis of ATP. The Na+/Ca2+ exchanger is secondary active. It exploits the Na+ ion gradient previously generated by the Na+/K+-ATPase, another primary active transport. Thus, both Ca2+ extrusion pathways require hydrolysis of ~1 ATP for the extrusion of 1 Ca2+ ion. e | Use of a small number of Ca2+ channels introduces stochastic components in Ca2+ channel opening and closing, without affecting the rising phase of corresponding Ca2+ transients. Main plot, simulated Ca2+ concentration 12 nm away from a single Ca2+ channel activated by an action potential. Inset, open probability of the single Ca2+ channel. Ten individual openings are shown superimposed. Red curves show a regime with an infinite number of Ca2+ channels for comparison. Note that the rising phases of the Ca2+ transients are similar, despite stochastic Ca2+ channel opening. Thus, the opening of the Ca2+ channels is stochastic, whereas the rising phase of the Ca2+ transients is largely deterministic. f | Use of a small number of Ca2+ channels may lead to excessive miniature release due to stochastic Ca2+ channel opening. Upper schematics, spontaneous opening of presynaptic Ca2+ channels; lower schematics, hypothetical ‘spontaneous’ release events driven by channel openings. Parts a–c are reproduced, with permission, from REF. © (2008) Elsevier. Part e is reproduced, with permission, from REF. © (2006) Macmillan Publishers Ltd. All rights reserved. In b and c, transmitter release was simulated using a previously established release model-.
Similar articles
- Loose coupling between Ca2+ channels and release sensors at a plastic hippocampal synapse.
Vyleta NP, Jonas P. Vyleta NP, et al. Science. 2014 Feb 7;343(6171):665-70. doi: 10.1126/science.1244811. Science. 2014. PMID: 24503854 - Developmental transformation of the release modality at the calyx of Held synapse.
Fedchyshyn MJ, Wang LY. Fedchyshyn MJ, et al. J Neurosci. 2005 Apr 20;25(16):4131-40. doi: 10.1523/JNEUROSCI.0350-05.2005. J Neurosci. 2005. PMID: 15843616 Free PMC article. - Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse.
Jarsky T, Tian M, Singer JH. Jarsky T, et al. J Neurosci. 2010 Sep 8;30(36):11885-95. doi: 10.1523/JNEUROSCI.1415-10.2010. J Neurosci. 2010. PMID: 20826653 Free PMC article. - The Nanophysiology of Fast Transmitter Release.
Stanley EF. Stanley EF. Trends Neurosci. 2016 Mar;39(3):183-197. doi: 10.1016/j.tins.2016.01.005. Epub 2016 Feb 16. Trends Neurosci. 2016. PMID: 26896416 Review. - The voltage-gated calcium channel functions as the molecular switch of synaptic transmission.
Atlas D. Atlas D. Annu Rev Biochem. 2013;82:607-35. doi: 10.1146/annurev-biochem-080411-121438. Epub 2013 Jan 17. Annu Rev Biochem. 2013. PMID: 23331239 Review.
Cited by
- Synapsin II desynchronizes neurotransmitter release at inhibitory synapses by interacting with presynaptic calcium channels.
Medrihan L, Cesca F, Raimondi A, Lignani G, Baldelli P, Benfenati F. Medrihan L, et al. Nat Commun. 2013;4:1512. doi: 10.1038/ncomms2515. Nat Commun. 2013. PMID: 23443540 Free PMC article. - Inter-channel scaffolding of presynaptic CaV2.2 via the C terminal PDZ ligand domain.
Gardezi SR, Li Q, Stanley EF. Gardezi SR, et al. Biol Open. 2013 Apr 9;2(5):492-8. doi: 10.1242/bio.20134267. Print 2013 May 15. Biol Open. 2013. PMID: 23789098 Free PMC article. - Mechanisms controlling the trafficking, localization, and abundance of presynaptic Ca2+ channels.
Cunningham KL, Littleton JT. Cunningham KL, et al. Front Mol Neurosci. 2023 Jan 13;15:1116729. doi: 10.3389/fnmol.2022.1116729. eCollection 2022. Front Mol Neurosci. 2023. PMID: 36710932 Free PMC article. Review. - Presynaptic calcium channels: specialized control of synaptic neurotransmitter release.
Dolphin AC, Lee A. Dolphin AC, et al. Nat Rev Neurosci. 2020 Apr;21(4):213-229. doi: 10.1038/s41583-020-0278-2. Epub 2020 Mar 11. Nat Rev Neurosci. 2020. PMID: 32161339 Free PMC article. Review. - Exocytotic machineries of vestibular type I and cochlear ribbon synapses display similar intrinsic otoferlin-dependent Ca2+ sensitivity but a different coupling to Ca2+ channels.
Vincent PF, Bouleau Y, Safieddine S, Petit C, Dulon D. Vincent PF, et al. J Neurosci. 2014 Aug 13;34(33):10853-69. doi: 10.1523/JNEUROSCI.0947-14.2014. J Neurosci. 2014. PMID: 25122888 Free PMC article.
References
- Katz B, Miledi R. The measurement of synaptic delay, and the time course of acetylcholine release at the neuromuscular junction. Proc. R. Soc. Lond. B. 1965;161:483–495. - PubMed
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
- Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996;384:170–172. - PubMed
- Geiger JRP, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous