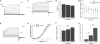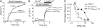Heteromeric heat-sensitive transient receptor potential channels exhibit distinct temperature and chemical response - PubMed (original) (raw)
Heteromeric heat-sensitive transient receptor potential channels exhibit distinct temperature and chemical response
Wei Cheng et al. J Biol Chem. 2012.
Abstract
TRPV1 and TRPV3 are two heat-sensitive ion channels activated at distinct temperature ranges perceived by human as hot and warm, respectively. Compounds eliciting human sensations of heat or warmth can also potently activate these channels. In rodents, TRPV3 is expressed predominantly in skin keratinocytes, whereas in humans TRPV1 and TRPV3 are co-expressed in sensory neurons of dorsal root ganglia and trigeminal ganglion and are known to form heteromeric channels with distinct single channel conductances as well as sensitivities to TRPV1 activator capsaicin and inhibitor capsazepine. However, how heteromeric TRPV1/TRPV3 channels respond to heat and other stimuli remains unknown. In this study, we examined the behavior of heteromeric TRPV1/TRPV3 channels activated by heat, capsaicin, and voltage. Our results demonstrate that the heteromeric channels exhibit distinct temperature sensitivity, activation threshold, and heat-induced sensitization. Changes in gating properties apparently originate from interactions between TRPV1 and TRPV3 subunits. Our results suggest that heteromeric TRPV1/TRPV3 channels are unique heat sensors that may contribute to the fine-tuning of sensitivity to sensory inputs.
Figures
FIGURE 1.
Heteromeric TRPV1/TRPV3 channels exhibit intermediate single channel conductance. A, sequence alignment between the pore regions of TRPV1 and TRPV3. B, representative single channel current traces recorded from cells expressing TRPV1, TRPV3, or TRPV1-TRPV3 concatemer at +80 mV. All-point histograms of current amplitude for each channel type are superimposed with fits of a double-Gaussian function. C, single channel conductance of TRPV1/TRPV3 concatemers (118.6 ± 6.9 picosiemens (pS), n = 6) is between those of homomeric TRPV1 (99.0 ± 3.9 picosiemens (n = 6) and TRPV3 (175.7 ± 4.6 picosiemens, n = 4) determined at +80 mV. *, p < 0.05; **, p < 0.01.
FIGURE 2.
Heteromeric TRPV1/TRPV3 channels exhibit altered capsaicin sensitivity. A and B, representative current traces of TRPV1 (A) and TRPV1/TRPV3 concatemer (B), recorded at +80 mV in response to different concentrations of capsaicin. C, capsaicin dose-response curves of TRPV1/TRPV3 (open squares) and TRPV1 (filled squares). The curves represent fits to the Hill equation. The EC50 and slope factor values are as follows: 269 ± 27 n
m
and 0.85 ± 0.06 (n = 6) for TRPV1/TRPV3; 981 ± 59 n
m
and 1.88 ± 0.07 (n = 3) for TRPV1. D, comparison of Hill coefficient (left panel) and EC50 (right panel) of TRPV1-TRPV1 and TRPV1-TRPV3 channels (normalized to TRPV1, n = 6 each). E, example of single channel traces of TRPV1 and TRPV1-TRPV3 channels in response to 10 μ
m
capsaicin (left panel). The bar graph represents mean open probability values (n = 3 for TRPV1; n = 4 for TRPV1-TRPV3). F, model of capsaicin activation. Gray triangles represents capsaicin molecule. TRPV1 subunits are illustrated as open circles, and TRPV3 subunits are shown as hatched circles. *, p < 0.05; **, p < 0.01.
FIGURE 3.
Voltage dependence properties of homomeric TRPV1, TRPV3, and heteromeric TRPV1/TRPV3 channel. A–C, representative macroscopic currents from TRPV1, TRPV3, and TRPV1-TRPV3 concatemer in response to voltage steps to −100 to +220 mV for 150 ms from a holding potential of 0 mV. D, G-V curves fitted to a Boltzmann function. For homomeric TRPV1, _V_half = 146 ± 19 mV, q = 0.53 ± 0.03 _e_0 (n = 5); for homomeric TRPV3, _V_half = 130 ± 7 mV, q = 0.68 ± 0.02 _e_0 (n = 7); for TRPV1-TRPV3 concatemer, _V_half = 138 ± 11 mV, q = 0.59 ± 0.05 _e_0 (n = 7). E, comparison of the _V_half values. F, comparison of the apparent gating charge q values. G, time constants of voltage-dependent activation of homomeric TRPV1 (n = 4), TRPV3 (n = 3–5), and TRPV1-TRPV3 concatemer (n = 5–7) measured at different voltages. H, time constants of voltage-dependent deactivation of homomeric TRPV1 (n = 4), TRPV3 (n = 5), and TRPV1-TRPV3 concatemer (n = 8) determined at −120 mV. **, p < 0.01.
FIGURE 4.
Shifts in voltage dependence caused by capsazepine. A and B, G-V curves of TRPV1-TRPV3 concatemer and homomeric TRPV1, respectively, in the presence or absence of 1 μ
m
capsazepine. Dotted curves represent fits of a Boltzmann function to data points. Dotted lines with an arrowhead in A indicate the _V_half values. C and _D, V_half values of TRPV1-TRPV3 concatemer and homomeric TRPV1, respectively (n = 3–7). E and F, capsazepine on and off rates for homomeric TRPV1, TRPV1-TRPV3 concatemer, and TRPV1 + TRPV3 co-expression (n = 3–9). *, p < 0.05; **, p < 0.01.
FIGURE 5.
Heteromeric TRPV1/TRPV3 channels share the same conducting pore. A, capsaicin-induced increases in intracellular Ca2+ concentration ([Ca2+]i) in cells expressing TRPV1 and TRPV1/TRPV3 concatemer. pcDNA3 was transfected as a negative control. B, co-expression of TRPV1 and TRPV3D641N caused a decrease in capsaicin-induced Ca2+ release and an increase in the agonist-induced Ca2+ influx. C, co-expression with TRPV3D641N, but not the wild type TRPV3, decreased the RR sensitivity of the capsaicin-induced Ca2+ response.
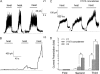
FIGURE 6.
TRPV1, TRPV3, and heteromeric TRPV1/TRPV3 channels exhibit distinct heat activation thresholds. A, representative heat activation time courses recorded at 80 mV. The temperature threshold is defined as the intersection (arrows) of the pair of fitted linear functions to the leak current and the heat-activated channel current. B, comparison of the measured temperature threshold values of homomeric TRPV1 (37.7 ± 0.3 °C, n = 9), TRPV1-TRPV1 concatemer (38.3 ± 0.5 °C, n = 6), TRPV3 (31.3 ± 0.6 °C, n = 7), TRPV1 + TRPV3 co-expression (33.7 ± 0.4 °C, n = 6), and TRPV1-TRPV3 concatemer (33.3 ± 0.6 °C, n = 10). *, p < 0.05; **, p < 0.01; n.s., not significant.
FIGURE 7.
TRPV1, TRPV3, and heteromeric TRPV1/TRPV3 channels exhibit distinct heat activation kinetics. A, representative current traces at +80 mV elicited by rapid laser heating (TRPV1, TRPV1 + TRPV3 co-expression, and TRPV1-TRPV3 concatemer) or by perfusion (TRPV3, because of its slow activation time course). A single exponential function is used to fit to the current raising phase (dotted curve) from which the time constant of heat activation is estimated. B, distribution of heat activation time constants. Values for TRPV1, TRPV1 + TRPV3 co-expression, and TRPV1-TRPV3 concatemer are measured from current responses to heating at 43 °C; those of TRPV3 are measured at 37 °C. Each symbol represents an individual measurement. C, temperature dependence of heat activation time constants of TRPV1 (n = 4–6 for each temperature level) and TRPV1/TRPV3 concatemer (n = 3–4). Dotted lines represent fits to the Arrhenius equation.
FIGURE 8.
Potentiation of TRPV1, TRPV3, and heteromeric TRPV1/TRPV3 channel by repetitive heating. A–C, representative current responses of homomeric TRPV1, homomeric TRPV3, and TRPV1/TRPV3 concatemer, respectively, to repeated heating up to 40 °C. D, fold change in current amplitude upon repeated heating (n = 3–8). *, p < 0.05.
Similar articles
- Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro.
St Pierre M, Reeh PW, Zimmermann K. St Pierre M, et al. Exp Brain Res. 2009 Jun;196(1):31-44. doi: 10.1007/s00221-009-1808-3. Epub 2009 Apr 30. Exp Brain Res. 2009. PMID: 19404626 Review. - Molecular cloning and functional characterization of Xenopus tropicalis frog transient receptor potential vanilloid 1 reveal its functional evolution for heat, acid, and capsaicin sensitivities in terrestrial vertebrates.
Ohkita M, Saito S, Imagawa T, Takahashi K, Tominaga M, Ohta T. Ohkita M, et al. J Biol Chem. 2012 Jan 20;287(4):2388-97. doi: 10.1074/jbc.M111.305698. Epub 2011 Nov 30. J Biol Chem. 2012. PMID: 22130664 Free PMC article. - Acute heat-evoked temperature sensation is impaired but not abolished in mice lacking TRPV1 and TRPV3 channels.
Marics I, Malapert P, Reynders A, Gaillard S, Moqrich A. Marics I, et al. PLoS One. 2014 Jun 12;9(6):e99828. doi: 10.1371/journal.pone.0099828. eCollection 2014. PLoS One. 2014. PMID: 24925072 Free PMC article. - Allyl isothiocyanate sensitizes TRPV1 to heat stimulation.
Alpizar YA, Boonen B, Gees M, Sanchez A, Nilius B, Voets T, Talavera K. Alpizar YA, et al. Pflugers Arch. 2014 Mar;466(3):507-15. doi: 10.1007/s00424-013-1334-9. Epub 2013 Aug 18. Pflugers Arch. 2014. PMID: 23955021 - Thermosensation and TRP Channels.
Tominaga M, Kashio M. Tominaga M, et al. Adv Exp Med Biol. 2024;1461:3-13. doi: 10.1007/978-981-97-4584-5_1. Adv Exp Med Biol. 2024. PMID: 39289270 Review.
Cited by
- Activation of temperature-sensitive TRPV1-like receptors in ARC POMC neurons reduces food intake.
Jeong JH, Lee DK, Liu SM, Chua SC Jr, Schwartz GJ, Jo YH. Jeong JH, et al. PLoS Biol. 2018 Apr 24;16(4):e2004399. doi: 10.1371/journal.pbio.2004399. eCollection 2018 Apr. PLoS Biol. 2018. PMID: 29689050 Free PMC article. - Involvement of calcium channels in the regulation of adipogenesis.
Zhai M, Yang D, Yi W, Sun W. Zhai M, et al. Adipocyte. 2020 Dec;9(1):132-141. doi: 10.1080/21623945.2020.1738792. Adipocyte. 2020. PMID: 32175809 Free PMC article. Review. - Channeling satiation: a primer on the role of TRP channels in the control of glutamate release from vagal afferent neurons.
Wu SW, Fenwick AJ, Peters JH. Wu SW, et al. Physiol Behav. 2014 Sep;136:179-84. doi: 10.1016/j.physbeh.2014.09.003. Epub 2014 Oct 5. Physiol Behav. 2014. PMID: 25290762 Free PMC article. Review. - TRPV3: time to decipher a poorly understood family member!
Nilius B, Bíró T, Owsianik G. Nilius B, et al. J Physiol. 2014 Jan 15;592(2):295-304. doi: 10.1113/jphysiol.2013.255968. Epub 2013 Jul 8. J Physiol. 2014. PMID: 23836684 Free PMC article. Review. - Selective inhibition of overactive warmth-sensitive Ca2+-permeable TRPV3 channels by antispasmodic agent flopropione for alleviation of skin inflammation.
Xu Y, Qu Y, Zhang C, Niu C, Tang X, Sun X, Wang K. Xu Y, et al. J Biol Chem. 2024 Feb;300(2):105595. doi: 10.1016/j.jbc.2023.105595. Epub 2023 Dec 26. J Biol Chem. 2024. PMID: 38154600 Free PMC article.
References
- Clapham D. E. (2002) Signal transduction. Hot and cold TRP ion channels. Science 295, 2228–2229 - PubMed
- Jordt S. E., McKemy D. D., Julius D. (2003) Lessons from peppers and peppermint. The molecular logic of thermosensation. Curr. Opin. Neurobiol. 13, 487–492 - PubMed
- Latorre R., Zaelzer C., Brauchi S. (2009) Structure-functional intimacies of transient receptor potential channels. Q. Rev. Biophys. 42, 201–246 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS072377-01A1/NS/NINDS NIH HHS/United States
- R01 GM081658/GM/NIGMS NIH HHS/United States
- GM081658/GM/NIGMS NIH HHS/United States
- R01 NS072377/NS/NINDS NIH HHS/United States
- NS072377/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources