Effects of cellular, chemical, and pharmacological chaperones on the rescue of a trafficking-defective mutant of the ATP-binding cassette transporter proteins ABCB1/ABCB4 - PubMed (original) (raw)
Effects of cellular, chemical, and pharmacological chaperones on the rescue of a trafficking-defective mutant of the ATP-binding cassette transporter proteins ABCB1/ABCB4
Julien Gautherot et al. J Biol Chem. 2012.
Abstract
The ATP-binding cassette transporter ABCB4 is a phosphatidylcholine translocator specifically expressed at the bile canalicular membrane in hepatocytes, highly homologous to the multidrug transporter ABCB1. Variations in the ABCB4 gene sequence cause progressive familial intrahepatic cholestasis type 3. We have shown previously that the I541F mutation, when reproduced either in ABCB1 or in ABCB4, led to retention in the endoplasmic reticulum (ER)/Golgi. Here, Madin-Darby canine kidney cells expressing ABCB1-GFP were used as a model to investigate this mutant. We show that ABCB1-I541F is not properly folded and is more susceptible to in situ protease degradation. It colocalizes and coprecipitates with the ER chaperone calnexin and coprecipitates with the cytosolic chaperone Hsc/Hsp70. Silencing of calnexin or overexpression of Hsp70 have no effect on maturation of the mutant. We also tested potential rescue by chemical and pharmacological chaperones. Thapsigargin and sodium 4-phenyl butyrate were inefficient. Glycerol improved maturation and exit of the mutant from the ER. Cyclosporin A, a competitive substrate for ABCB1, restored maturation, plasma membrane expression, and activity of ABCB1-I541F. Cyclosporin A also improved maturation of ABCB4-I541F in Madin-Darby canine kidney cells. In HepG(2) cells transfected with ABCB4-I541F cDNA, cyclosporin A allowed a significant amount of the mutant protein to reach the membrane of bile canaliculi. These results show that the best strategy to rescue conformation-defective ABCB4 mutants is provided by pharmacological chaperones that specifically target the protein. They identify cyclosporin A as a potential novel therapeutic tool for progressive familial intrahepatic cholestasis type 3 patients.
Figures
FIGURE 1.
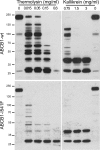
In situ protease susceptibility of ABCB1-WT and ABCB1-I541F. Microsomes obtained from MDCK cells expressing GFP-tagged ABCB1-WT or ABCB1-I541F were submitted to limited proteolysis at the indicated concentrations of thermolysin or kallikrein for 30 min at 4 °C. Samples (ABCB1, 30 μg and ABCB1-I541F, 50 μg protein per lane) were immunoblotted with the anti-GFP monoclonal antibody. Molecular mass standards are indicated in kDa.
FIGURE 2.
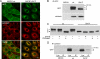
ABCB1-I541F colocalizes and coprecipitates with calnexin. A, MDCK cells stably transfected with GFP-tagged ABCB1-WT or ABCB1-I541F were fixed, and immunolocalization of calnexin was performed using a Cy3-conjugated secondary antibody. Confocal images show strong colocalization of the mutant with calnexin. Note that the staining pattern of calnexin in ABCB1-I541F cells is different from that in ABCB1-WT cells because of ER accumulation of the mutant. Scale bars = 10 μm. B, MDCK cells stably transfected with GFP-tagged ABCB1-WT or ABCB1-I541F and control MDCK cells were lysed, and immunoprecipitation (IP) was performed with the anti-GFP antibody. Samples were analyzed by immunoblotting using anti-GFP or anti-calnexin monoclonal antibodies. C, cell lysates were subjected to endoH or peptide _N_-glycosidase F digestion and processed for immunoblotting using the anti-GFP antibody. The mature (A), immature (B) and deglycosylated (C) forms are indicated. D, MDCK cells stably transfected with GFP-tagged ABCB1-WT or ABCB1-I541F and control MDCK cells were lysed, and immunoprecipitation was performed with the anti-calnexin antibody. Samples were analyzed by immunoblotting using the anti-GFP antibody. Lys, whole cell lysates.
FIGURE 3.
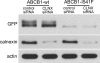
Effect of calnexin silencing on ABCB1-I541F expression. MDCK cells stably transfected with GFP-tagged ABCB1-WT or ABCB1-I541F were transfected with control or calnexin (CLNX) siRNA. After 72 h, cells were lysed, and samples were analyzed by immunoblotting using anti-GFP or anti-calnexin antibodies. Actin was taken as an internal standard. Shown is one representative of three experiments.
FIGURE 4.
ABCB1-I541F coprecipitates with Hsc70. A, MDCK cells stably transfected with GFP-tagged ABCB1-I541F were fixed, and Hsc70 was detected by immunofluorescence using a Cy3-conjugated secondary antibody. Images were obtained by confocal microscopy. Scale bar = 10 μm. B, MDCK cells stably transfected with GFP-tagged ABCB1-WT or ABCB1-I541F and control MDCK cells were lysed, and immunoprecipitation (IP) was performed using the monoclonal anti-GFP antibody. Samples were analyzed by immunoblotting using anti-GFP or anti-Hsc70 monoclonal antibodies. Shown is one representative of three experiments. Lys, whole cell lysates.
FIGURE 5.
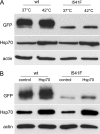
Effect of Hsp70 overexpression on ABCB1-I541F expression. A, MDCK cells stably transfected with GFP-tagged ABCB1-WT or ABCB1-I541F were maintained at 37 °C or exposed at 42 °C for 2 h and then returned to 37 °C for 24 h. Cells were lysed, and samples were analyzed by immunoblotting using anti-GFP and anti-Hsp70 antibodies. Shown is one representative of three experiments. B, MDCK cells stably transfected with GFP-tagged ABCB1-WT or ABCB1-I541F were transfected with the plasmid encoding Hsp70. After 48 h, cells were analyzed by immunoblotting using anti-GFP and anti-Hsp70 antibodies. Actin was taken as an internal standard. Shown is one representative of four experiments.
FIGURE 6.
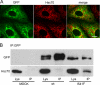
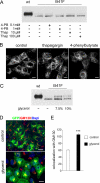
Effect of chemical chaperones on the expression pattern of ABCB1-I541F. A, MDCK cells stably transfected with GFP-tagged ABCB1-WT or ABCB1-I541F were treated with vehicle (-), 4-PB, or thapsigargin (Thap) at the indicated concentrations for 24 h. Immunoblotting was performed with anti-GFP. B, filter-grown MDCK cells stably transfected with GFP-tagged ABCB1-I541F were treated with vehicle (control), 100 μ
m
thapsigargin, or 1 m
m
4-phenylbutyrate for 24 h. Cells were fixed, and GFP fluorescence was analyzed by confocal microscopy. Scale bars = 10 μm. C, the same experiment as in A, except that cells were treated with 7.5% or 10% glycerol (w/v). D, MDCK cells stably transfected with GFP-tagged ABCB1-I541F were treated with 7.5% glycerol for 24 h. Cells were fixed and stained with the anti-GM130 antibody and a Cy3-conjugated secondary antibody. Nuclei were stained with DAPI. Images were obtained by confocal microscopy. Scale bar = 10 μm. E, the colocalization of GFP-tagged ABCB1-I541F with GM130 was quantified using the ImageJ 1.41 measure colocalization function on multiple confocal sections of at least 30 cells in three independent experiments. Open bar, control cells; black bar, glycerol-treated cells. Data (arbitrary units) are expressed as mean ± S.E. ***, p < 0.001.
FIGURE 7.
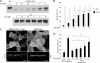
Cyclosporin A rescues ABCB1-I541F. MDCK cells stably transfected with GFP-tagged ABCB1-WT or ABCB1-I541F were treated with vehicle or cyclosporin A (CsA) at the indicated concentrations for 24 h. A, homogenates were analyzed by immunoblotting using the anti-GFP monoclonal antibody. B, quantification of experiments shown in A. The mature and immature bands were separately quantified on gels, and the relative amount of the mature form for each condition was calculated (mean ± S.D. of three experiments). C, confocal microscopy of MDCK cells stably transfected with GFP-tagged ABCB1-I541F and treated with 10 μ
m
cyclosporin A (a and a') and MDCK cells stably transfected with GFP-tagged ABCB1-WT (b and b'). a and b are xy projections. xz sections (a' and b') show exclusive apical staining. Brackets indicate the height of the monolayer. Scale bars = 10 μm. D, control MDCK cells and MDCK cells stably transfected with GFP-tagged ABCB1-wt, or ABCB1-I541F were grown at 37 °C in 96-wells plates for 3 days. The cells were treated with the indicated concentrations of cyclosporin A 24 h before the calcein assay was performed. Results are expressed as the percentage of fluorescent calcein extruded after 60 min (mean ± S.D. of four determinations performed in triplicate). *, p < 0.05); **, p < 0.01; ***, p < 0.001.
FIGURE 8.
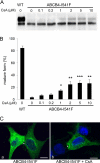
Cyclosporin A rescues ABCB4-I541F. A, MDCK cells were transfected with plasmids encoding ABCB4-WT or ABCB4-I541F. Six hours later, cyclosporin A (CsA) was added at the indicated concentrations. After 24 h, cells were lysed, and samples (40 μg protein) were analyzed by immunoblotting with an anti-ABCB4 antibody. B, quantification of experiments shown in A. The mature and immature bands were separately quantified on gels, and the relative amount of the mature form for each condition was calculated (mean ± S.D. of three experiments). *, p < 0.05); **, p < 0.01); ***, p < 0.001. C, HepG2 cells transfected with the pCDNA3-ABCB4 plasmid were fixed, and ABCB4 was detected by immunofluorescence using an Alexa Fluor 488 secondary antibody. a, control cells; b, cells treated with 5 μ
m
cyclosporin A for 18 h. Images are projections of focal sections obtained by confocal microscopy. Nuclei were stained with DAPI. The asterisks indicate the location of bile canaliculi. Scale bars = 10 μm.
Similar articles
- A missense mutation in ABCB4 gene involved in progressive familial intrahepatic cholestasis type 3 leads to a folding defect that can be rescued by low temperature.
Delaunay JL, Durand-Schneider AM, Delautier D, Rada A, Gautherot J, Jacquemin E, Aït-Slimane T, Maurice M. Delaunay JL, et al. Hepatology. 2009 Apr;49(4):1218-27. doi: 10.1002/hep.22775. Hepatology. 2009. PMID: 19185004 - Structural analogues of roscovitine rescue the intracellular traffic and the function of ER-retained ABCB4 variants in cell models.
Vauthier V, Ben Saad A, Elie J, Oumata N, Durand-Schneider AM, Bruneau A, Delaunay JL, Housset C, Aït-Slimane T, Meijer L, Falguières T. Vauthier V, et al. Sci Rep. 2019 Apr 30;9(1):6653. doi: 10.1038/s41598-019-43111-y. Sci Rep. 2019. PMID: 31040306 Free PMC article. - A functional classification of ABCB4 variations causing progressive familial intrahepatic cholestasis type 3.
Delaunay JL, Durand-Schneider AM, Dossier C, Falguières T, Gautherot J, Davit-Spraul A, Aït-Slimane T, Housset C, Jacquemin E, Maurice M. Delaunay JL, et al. Hepatology. 2016 May;63(5):1620-31. doi: 10.1002/hep.28300. Epub 2015 Dec 23. Hepatology. 2016. PMID: 26474921 - ABCB4 Gene Aberrations in Human Liver Disease: An Evolving Spectrum.
Reichert MC, Lammert F. Reichert MC, et al. Semin Liver Dis. 2018 Nov;38(4):299-307. doi: 10.1055/s-0038-1667299. Epub 2018 Oct 24. Semin Liver Dis. 2018. PMID: 30357767 Review. - Variants in ABCB4 (MDR3) across the spectrum of cholestatic liver diseases in adults.
Stättermayer AF, Halilbasic E, Wrba F, Ferenci P, Trauner M. Stättermayer AF, et al. J Hepatol. 2020 Sep;73(3):651-663. doi: 10.1016/j.jhep.2020.04.036. Epub 2020 May 4. J Hepatol. 2020. PMID: 32376413 Review.
Cited by
- P-glycoprotein is fully active after multiple tryptophan substitutions.
Swartz DJ, Weber J, Urbatsch IL. Swartz DJ, et al. Biochim Biophys Acta. 2013 Mar;1828(3):1159-68. doi: 10.1016/j.bbamem.2012.12.005. Epub 2012 Dec 19. Biochim Biophys Acta. 2013. PMID: 23261390 Free PMC article. - Phenylalanine hydroxylase misfolding and pharmacological chaperones.
Underhaug J, Aubi O, Martinez A. Underhaug J, et al. Curr Top Med Chem. 2012;12(22):2534-45. doi: 10.2174/1568026611212220008. Curr Top Med Chem. 2012. PMID: 23339306 Free PMC article. Review. - Functional Characterization of ABCB4 Mutations Found in Low Phospholipid-Associated Cholelithiasis (LPAC).
Kim TH, Park HJ, Choi JH. Kim TH, et al. Korean J Physiol Pharmacol. 2013 Dec;17(6):525-30. doi: 10.4196/kjpp.2013.17.6.525. Epub 2013 Dec 16. Korean J Physiol Pharmacol. 2013. PMID: 24381502 Free PMC article. - Functional Rescue of Trafficking-Impaired ABCB4 Mutants by Chemical Chaperones.
Gordo-Gilart R, Andueza S, Hierro L, Jara P, Alvarez L. Gordo-Gilart R, et al. PLoS One. 2016 Feb 22;11(2):e0150098. doi: 10.1371/journal.pone.0150098. eCollection 2016. PLoS One. 2016. PMID: 26900700 Free PMC article. - Molecular Regulation of Canalicular ABC Transporters.
Ben Saad A, Bruneau A, Mareux E, Lapalus M, Delaunay JL, Gonzales E, Jacquemin E, Aït-Slimane T, Falguières T. Ben Saad A, et al. Int J Mol Sci. 2021 Feb 20;22(4):2113. doi: 10.3390/ijms22042113. Int J Mol Sci. 2021. PMID: 33672718 Free PMC article. Review.
References
- Hebert D. N., Molinari M. (2007) In and out of the ER. Protein folding, quality control, degradation, and related human diseases. Physiol. Rev. 87, 1377–1408 - PubMed
- Määttänen P., Gehring K., Bergeron J. J., Thomas D. Y. (2010) Protein quality control in the ER. The recognition of misfolded proteins. Semin. Cell Dev. Biol. 21, 500–511 - PubMed
- Kleizen B., Braakman I. (2004) Protein folding and quality control in the endoplasmic reticulum. Curr. Opin. Cell Biol. 16, 343–349 - PubMed
- Meusser B., Hirsch C., Jarosch E., Sommer T. (2005) ERAD. The long road to destruction. Nat. Cell Biol. 7, 766–772 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous