Transfusion of older stored blood and risk of death: a meta-analysis - PubMed (original) (raw)
Review
Transfusion of older stored blood and risk of death: a meta-analysis
Dong Wang et al. Transfusion. 2012 Jun.
Abstract
Background: Blood for transfusion is stored for up to 42 days. Older blood develops lesions and accumulates potentially injurious substances. Some studies report increasing toxicity as blood ages. We assessed the safety of transfused older versus newer stored blood.
Study design and methods: PubMed, Scopus, and Embase were searched using terms new and old and red blood cell and storage through May 6, 2011, for observational and randomized controlled studies comparing outcomes using transfused blood having longer and shorter storage times. Death was the outcome of interest.
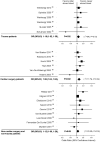
Results: Twenty-one studies were identified, predominantly in cardiac surgery (n=6) and trauma (n=6) patients, including 409,966 patients. A test for heterogeneity of these studies' results was not significant for mortality (I(2)=3.7%, p=0.41). Older blood was associated with a significantly increased risk of death (odds ratio, 1.16; 95% confidence interval [CI], 1.07-1.24). Using available mortality data, 97 (95% CI, 63-199) patients need to be treated with only new blood to save one life. Subgroup analysis of these trials indicated that the increased risk was not restricted to a particular type of patient, size of trial, or amount of blood transfused.
Conclusion: Based on available data, use of older stored blood is associated with a significantly increased risk of death.
© 2011 American Association of Blood Banks.
Figures
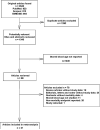
Figure 1
Study selection
Figure 2. Funnel plot of studies included in meta-analysis
Each open circle represents one of 21 studies in our meta-analysis. The precision for each study is plotted versus log (OR). The circles are distributed equally around the solid vertical line with a solid diamond at the bottom, representing the overall treatment effect in this study. There is no skewed distribution of studies giving evidence for a publication bias. Eggers test for publication bias (or small study effect) is non-significant (P=0.43) and confirm what is seen on visual expectedness.
Figure 3. Mortality
The size of the data markers is proportional to the inverse variance of each point estimate.
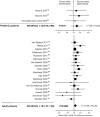
Figure 4
Severe adverse effects.
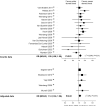
Figure 5
Subgroup analysis (Patient types).
Figure 6
Subgroup analysis (Pediatric and Adult).
Figure 7
Subgroup analysis (Adjusted mortality versus counts).
Comment in
- Old blood bad? Either the biggest issue in transfusion medicine or a nonevent.
Warkentin TE, Eikelboom JW. Warkentin TE, et al. Transfusion. 2012 Jun;52(6):1165-7. doi: 10.1111/j.1537-2995.2012.03671.x. Transfusion. 2012. PMID: 22686530 No abstract available. - Transfusion of older blood and risk of death: unanswered questions.
Cheuk DK. Cheuk DK. Transfusion. 2012 Jul;52(7):1595-6. doi: 10.1111/j.1537-2995.2012.03577.x. Transfusion. 2012. PMID: 22780898 No abstract available.
Similar articles
- Transfusion of recently donated (fresh) red blood cells (RBCs) does not improve survival in comparison with current practice, while safety of the oldest stored units is yet to be established: a meta-analysis.
Remy KE, Sun J, Wang D, Welsh J, Solomon SB, Klein HG, Natanson C, Cortés-Puch I. Remy KE, et al. Vox Sang. 2016 Jul;111(1):43-54. doi: 10.1111/vox.12380. Epub 2016 Feb 5. Vox Sang. 2016. PMID: 26848822 Free PMC article. - The age of blood in pediatric intensive care units (ABC PICU): study protocol for a randomized controlled trial.
Tucci M, Lacroix J, Fergusson D, Doctor A, Hébert P, Berg RA, Caro J, Josephson CD, Leteurtre S, Menon K, Schechtman K, Steiner ME, Turgeon AF, Clayton L, Bockelmann T, Spinella PC; Canadian Critical Care Trials Group; Pediatric Critical Care Blood Research Network (BloodNet); Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Tucci M, et al. Trials. 2018 Jul 28;19(1):404. doi: 10.1186/s13063-018-2809-y. Trials. 2018. PMID: 30055634 Free PMC article. - Longer RBC storage duration is associated with increased postoperative infections in pediatric cardiac surgery.
Cholette JM, Pietropaoli AP, Henrichs KF, Alfieris GM, Powers KS, Phipps R, Spinelli SL, Swartz M, Gensini F, Daugherty LE, Nazarian E, Rubenstein JS, Sweeney D, Eaton M, Blumberg N. Cholette JM, et al. Pediatr Crit Care Med. 2015 Mar;16(3):227-35. doi: 10.1097/PCC.0000000000000320. Pediatr Crit Care Med. 2015. PMID: 25607740 Free PMC article. Clinical Trial. - [Clinical impact of length of storage before red blood cell transfusion].
Lacroix J, Tucci M. Lacroix J, et al. Transfus Clin Biol. 2011 Apr;18(2):97-105. doi: 10.1016/j.tracli.2011.02.020. Epub 2011 Apr 3. Transfus Clin Biol. 2011. PMID: 21459646 Review. French. - In vitro assays and clinical trials in red blood cell aging: Lost in translation.
Prudent M, Tissot JD, Lion N. Prudent M, et al. Transfus Apher Sci. 2015 Jun;52(3):270-6. doi: 10.1016/j.transci.2015.04.006. Epub 2015 Apr 16. Transfus Apher Sci. 2015. PMID: 25982219 Review.
Cited by
- Cryopreserved packed red blood cells in surgical patients: past, present, and future.
Chang A, Kim Y, Hoehn R, Jernigan P, Pritts T. Chang A, et al. Blood Transfus. 2017 Jul;15(4):341-347. doi: 10.2450/2016.0083-16. Epub 2016 Sep 8. Blood Transfus. 2017. PMID: 27643751 Free PMC article. Review. - Storage with ethanol attenuates the red blood cell storage lesion.
Zingg SW, Schuster R, Joseph B, Caldwell CC, Lentsch AB, Goodman MD, Pritts TA. Zingg SW, et al. Surgery. 2022 Dec;172(6):1829-1836. doi: 10.1016/j.surg.2022.07.016. Epub 2022 Sep 13. Surgery. 2022. PMID: 36109200 Free PMC article. - Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia.
Solomon SB, Wang D, Sun J, Kanias T, Feng J, Helms CC, Solomon MA, Alimchandani M, Quezado M, Gladwin MT, Kim-Shapiro DB, Klein HG, Natanson C. Solomon SB, et al. Blood. 2013 Feb 28;121(9):1663-72. doi: 10.1182/blood-2012-10-462945. Epub 2012 Dec 18. Blood. 2013. PMID: 23255558 Free PMC article. - Measurement of red cell lifespan and aging.
Franco RS. Franco RS. Transfus Med Hemother. 2012 Oct;39(5):302-7. doi: 10.1159/000342232. Epub 2012 Aug 27. Transfus Med Hemother. 2012. PMID: 23801920 Free PMC article. - Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease.
Gladwin MT, Kanias T, Kim-Shapiro DB. Gladwin MT, et al. J Clin Invest. 2012 Apr;122(4):1205-8. doi: 10.1172/JCI62972. Epub 2012 Mar 26. J Clin Invest. 2012. PMID: 22446184 Free PMC article.
References
- World Health Organization . Global Database on Blood Safety: Report 2004-2005. World Health Organization; Geneva, Swizerland: 2008.
- Whitaker BI, Schlumpf K Schulman J, Green J. The 2009 Nationwide Blood Collection and Utilization Survey Report. Besthesda, MD: 2011. ISBN.
- Hill HR, Oliver CK, Lippert LE, Greenwalt TJ, Hess JR. The effects of polyvinyl chloride and polyolefin blood bags on red blood cells stored in a new additive solution. Vox Sang. 2001;81(3):161–166. - PubMed
- Heaton WA, Holme S, Smith K, et al. Effects of 3-5 log10 pre-storage leucocyte depletion on red cell storage and metabolism. Br J Haematol. 1994;87(2):363–368. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical