MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults - PubMed (original) (raw)
MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults
Bradford C Dickerson et al. Neurology. 2012.
Abstract
Objective: New preclinical Alzheimer disease (AD) diagnostic criteria have been developed using biomarkers in cognitively normal (CN) adults. We implemented these criteria using an MRI biomarker previously associated with AD dementia, testing the hypothesis that individuals at high risk for preclinical AD would be at elevated risk for cognitive decline.
Methods: The Alzheimer's Disease Neuroimaging Initiative database was interrogated for CN individuals. MRI data were processed using a published set of a priori regions of interest to derive a single measure known as the AD signature (ADsig). Each individual was classified as ADsig-low (≥ 1 SD below the mean: high risk for preclinical AD), ADsig-average (within 1 SD of mean), or ADsig-high (≥ 1 SD above mean). A 3-year cognitive decline outcome was defined a priori using change in Clinical Dementia Rating sum of boxes and selected neuropsychological measures.
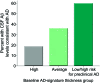
Results: Individuals at high risk for preclinical AD were more likely to experience cognitive decline, which developed in 21% compared with 7% of ADsig-average and 0% of ADsig-high groups (p = 0.03). Logistic regression demonstrated that every 1 SD of cortical thinning was associated with a nearly tripled risk of cognitive decline (p = 0.02). Of those for whom baseline CSF data were available, 60% of the high risk for preclinical AD group had CSF characteristics consistent with AD while 36% of the ADsig-average and 19% of the ADsig-high groups had such CSF characteristics (p = 0.1).
Conclusions: This approach to the detection of individuals at high risk for preclinical AD-identified in single CN individuals using this quantitative ADsig MRI biomarker-may provide investigators with a population enriched for AD pathobiology and with a relatively high likelihood of imminent cognitive decline consistent with prodromal AD.
Figures
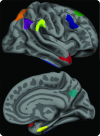
Figure 1. The cortical signature of Alzheimer disease (AD)
A priori regions of interest composing the “AD signature” in which consistent thinning has been previously observed in multiple samples of patients with mild AD dementia. The MRI biomarker used in the present study is an average of the thickness of the cerebral cortex in all 9 of these regions of interest, obtained from each individual subject.
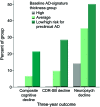
Figure 2. Expression of cortical signature of Alzheimer disease (AD) is associated with future cognitive decline
Participants who were cognitively normal at baseline but classified as high risk for preclinical AD on the basis of having low AD-signature cortical thickness were at markedly elevated risk of meeting the 3-year cognitive decline outcome (composite cognitive decline) as compared with participants with average or high AD-signature cortical thickness. Similar findings were present when the individual CDR–sum of boxes (CDR-SB) decline outcome or the neuropsychological performance decline outcome were examined.
Figure 3. Expression of cortical signature of Alzheimer disease (AD) is associated with AD-like spinal fluid
Cognitively normal participants who were classified as high risk for preclinical AD on the basis of having low AD-signature cortical thickness showed a trend toward being more likely to harbor abnormally low amyloid-β levels in CSF compared to participants with average or high AD-signature cortical thickness.
Comment in
- MRI-based biomarkers of preclinical AD: an Alzheimer signature.
Resnick SM, Scheltens P. Resnick SM, et al. Neurology. 2012 Jan 10;78(2):80-1. doi: 10.1212/WNL.0b013e31824237a5. Epub 2011 Dec 21. Neurology. 2012. PMID: 22189450 No abstract available.
Similar articles
- MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change.
Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR Jr; Alzheimer's Disease Neuroimaging Initiative. Vemuri P, et al. Neurology. 2009 Jul 28;73(4):294-301. doi: 10.1212/WNL.0b013e3181af79fb. Neurology. 2009. PMID: 19636049 Free PMC article. - Diagnostic and Prognostic Utility of the Synaptic Marker Neurogranin in Alzheimer Disease.
Tarawneh R, D'Angelo G, Crimmins D, Herries E, Griest T, Fagan AM, Zipfel GJ, Ladenson JH, Morris JC, Holtzman DM. Tarawneh R, et al. JAMA Neurol. 2016 May 1;73(5):561-71. doi: 10.1001/jamaneurol.2016.0086. JAMA Neurol. 2016. PMID: 27018940 Free PMC article. - MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations.
Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR Jr; Alzheimer's Disease Neuroimaging Initiative. Vemuri P, et al. Neurology. 2009 Jul 28;73(4):287-93. doi: 10.1212/WNL.0b013e3181af79e5. Neurology. 2009. PMID: 19636048 Free PMC article. - Evolving Evidence for the Value of Neuroimaging Methods and Biological Markers in Subjects Categorized with Subjective Cognitive Decline.
Lista S, Molinuevo JL, Cavedo E, Rami L, Amouyel P, Teipel SJ, Garaci F, Toschi N, Habert MO, Blennow K, Zetterberg H, O'Bryant SE, Johnson L, Galluzzi S, Bokde AL, Broich K, Herholz K, Bakardjian H, Dubois B, Jessen F, Carrillo MC, Aisen PS, Hampel H. Lista S, et al. J Alzheimers Dis. 2015 Sep 24;48 Suppl 1:S171-91. doi: 10.3233/JAD-150202. J Alzheimers Dis. 2015. PMID: 26402088 Review. - [Use of cerebrospinal fluid (CSF) biomarkers for Alzheimer's type dementia: diagnosis in mild cognitive impairment].
Verhey FR, Visser PJ. Verhey FR, et al. Ned Tijdschr Geneeskd. 2013;157(11):A5596. Ned Tijdschr Geneeskd. 2013. PMID: 23484512 Review. Dutch.
Cited by
- White matter hyperintensities are more highly associated with preclinical Alzheimer's disease than imaging and cognitive markers of neurodegeneration.
Kandel BM, Avants BB, Gee JC, McMillan CT, Erus G, Doshi J, Davatzikos C, Wolk DA. Kandel BM, et al. Alzheimers Dement (Amst). 2016 Apr 7;4:18-27. doi: 10.1016/j.dadm.2016.03.001. eCollection 2016. Alzheimers Dement (Amst). 2016. PMID: 27489875 Free PMC article. - An MRI measure of degenerative and cerebrovascular pathology in Alzheimer disease.
Brickman AM, Tosto G, Gutierrez J, Andrews H, Gu Y, Narkhede A, Rizvi B, Guzman V, Manly JJ, Vonsattel JP, Schupf N, Mayeux R. Brickman AM, et al. Neurology. 2018 Oct 9;91(15):e1402-e1412. doi: 10.1212/WNL.0000000000006310. Epub 2018 Sep 14. Neurology. 2018. PMID: 30217936 Free PMC article. - Distinct neural correlates of episodic memory among apolipoprotein E alleles in cognitively normal elderly.
Shu H, Shi Y, Chen G, Wang Z, Liu D, Yue C, Ward BD, Li W, Xu Z, Chen G, Guo QH, Xu J, Li SJ, Zhang Z. Shu H, et al. Brain Imaging Behav. 2019 Feb;13(1):255-269. doi: 10.1007/s11682-017-9818-4. Brain Imaging Behav. 2019. PMID: 29396739 Free PMC article. - Cortical atrophy patterns of incident MCI subtypes in the Mayo Clinic Study of Aging.
Machulda MM, Lundt ES, Albertson SM, Spychalla AJ, Schwarz CG, Mielke MM, Jack CR Jr, Kremers WK, Vemuri P, Knopman DS, Jones DT, Bondi MW, Petersen RC. Machulda MM, et al. Alzheimers Dement. 2020 Jul;16(7):1013-1022. doi: 10.1002/alz.12108. Epub 2020 May 17. Alzheimers Dement. 2020. PMID: 32418367 Free PMC article. - Investigating the temporal pattern of neuroimaging-based brain age estimation as a biomarker for Alzheimer's Disease related neurodegeneration.
Taylor A, Zhang F, Niu X, Heywood A, Stocks J, Feng G, Popuri K, Beg MF, Wang L; Alzheimer's Disease Neuroimaging Initiative. Taylor A, et al. Neuroimage. 2022 Nov;263:119621. doi: 10.1016/j.neuroimage.2022.119621. Epub 2022 Sep 9. Neuroimage. 2022. PMID: 36089183 Free PMC article.
References
- Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006; 66: 1837– 1844 - PubMed
- Morris JC, Storandt M, McKeel DW, Jr, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer's disease. Neurology 1996; 46: 707– 719 - PubMed
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging 1991; 12: 295– 312 - PubMed
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol 2001; 58: 1395– 1402 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG022374/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
- UL1 RR033173/RR/NCRR NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- P30AG010124/AG/NIA NIH HHS/United States
- R21-AG29840/AG/NIA NIH HHS/United States
- K01 AG030514/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- UL1 TR001998/TR/NCATS NIH HHS/United States
- K23-AG028018/AG/NIA NIH HHS/United States
- UL1 TR000117/TR/NCATS NIH HHS/United States
- P50-AG005134/AG/NIA NIH HHS/United States
- R01-AG29411/AG/NIA NIH HHS/United States
- R01 AG012101/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical