ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells - PubMed (original) (raw)
. 2012 Feb;180(2):599-607.
doi: 10.1016/j.ajpath.2011.10.036. Epub 2011 Dec 18.
Virginie Ory, Sandra Chapman, Hang Yuan, Chris Albanese, Bhaskar Kallakury, Olga A Timofeeva, Caitlin Nealon, Aleksandra Dakic, Vera Simic, Bassem R Haddad, Johng S Rhim, Anatoly Dritschilo, Anna Riegel, Alison McBride, Richard Schlegel
Affiliations
- PMID: 22189618
- PMCID: PMC3349876
- DOI: 10.1016/j.ajpath.2011.10.036
ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells
Xuefeng Liu et al. Am J Pathol. 2012 Feb.
Abstract
We demonstrate that a Rho kinase inhibitor (Y-27632), in combination with fibroblast feeder cells, induces normal and tumor epithelial cells from many tissues to proliferate indefinitely in vitro, without transduction of exogenous viral or cellular genes. Primary prostate and mammary cells, for example, are reprogrammed toward a basaloid, stem-like phenotype and form well-organized prostaspheres and mammospheres in Matrigel. However, in contrast to the selection of rare stem-like cells, the described growth conditions can generate 2 × 10(6) cells in 5 to 6 days from needle biopsies, and can generate cultures from cryopreserved tissue and from fewer than four viable cells. Continued cell proliferation is dependent on both feeder cells and Y-27632, and the conditionally reprogrammed cells (CRCs) retain a normal karyotype and remain nontumorigenic. This technique also efficiently establishes cell cultures from human and rodent tumors. For example, CRCs established from human prostate adenocarcinoma displayed instability of chromosome 13, proliferated abnormally in Matrigel, and formed tumors in mice with severe combined immunodeficiency. The ability to rapidly generate many tumor cells from small biopsy specimens and frozen tissue provides significant opportunities for cell-based diagnostics and therapeutics (including chemosensitivity testing) and greatly expands the value of biobanking. In addition, the CRC method allows for the genetic manipulation of epithelial cells ex vivo and their subsequent evaluation in vivo in the same host.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
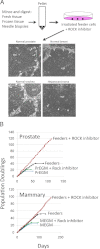
Propagation and immortalization of human adult epithelial cells. A: Prostate, breast, tracheal, and liver (hepatocellular carcinoma) tissues were harvested and digested with trypsin-collagenase, as described in Materials and Methods. Cells isolated from the tissues were plated on a feeder layer of irradiated (3000 rad) Swiss 3T3 cells (J2 subclone) and grown in F medium containing 10 μmol/L ROCK inhibitor (Y-27632). Small colonies could be observed after 1 day. At day 5 (shown), there were large islands of epithelial (epi) cells that compressed the surrounding feeder cells (white arrows). B: The prostate and breast epithelial cells were passaged repetitively using trypsinization techniques described for keratinocytes. The cell number was recorded at each passage, and a plot of population doublings versus time (days) was constructed. The cells were grown under four different conditions: with both feeder cells and Y-27632 in F medium, with feeders only in F medium, with Y-27632 in mammary epithelial growth medium or PrEGM or mammary epithelial growth medium or PrEGM alone. Only cells grown in F medium containing feeders and Y-27632 continued to proliferate with a constant growth rate.
Figure 2
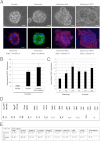
Characterization of late-passage breast and prostate cells. A: The differentiation potential of prostate and breast cells was evaluated by culture in Matrigel, without feeders or Y-27632, as described in Materials and Methods. Both prostate and breast cells formed normal prostaspheres (A, top row, left panel) and mammospheres (A, top row, second panel from left) with glandular lumens. The mammospheres showed normal polarized laminin deposition around the periphery of the sphere (A, bottom row, left panel). In addition, the mammospheres also showed the characteristic cytoplasmic/plasma membrane localization of β-catenin observed in normal epithelial cells (A, bottom row, second panel from left, green). In contrast, mammary cells immortalized with the Myc T58A mutant showed abnormal differentiation and formed abnormally shaped solid spheres with extensions into the Matrigel (A, top row, second panel from right). Scale bars: 25 μm (white); 20 μm (yellow). These Myc-immortalized cells also exhibited abnormal deposition of laminin within the sphere and without polarized localization to the colony periphery (A, bottom row, second panel from right). Mammary cells immortalized by hTERT also formed abnormal solid spheres (A, top row, right panel) with aberrant, dispersed distribution of laminin (A, bottom row, right panel). B: The cause of the high hTERT expression in the epithelial cells is the result of the in vitro culture conditions. When prostate cells are grown in commercial medium (PrEGM), the level of hTERT mRNA is low. However, when those cells are transferred to F medium with feeders (with or without Y-27632), there is an approximate 20-fold increase in hTERT expression. The greatest impact is the presence of feeder cells. C: Induction of hTERT mRNA in breast and prostate cells is similar and occurs early during passaging. Quantitative real-time PCR for hTERT was performed at the indicated passage numbers. Both cell types show an early induction of telomerase expression. In prostate, for example, the induction is maximal already at passage 2, indicating that selection during in vitro culture is not the cause of this induction. D: Chromosomal analysis of late-passage prostate cells revealed a normal 46,XY karyotype. E: DNA fingerprinting of early- and late-passage prostate cells demonstrated that they have nine identical STR loci and the Y-specific Amelogenin locus, thereby verifying their genetic identity. Data are presented as mean ± SEM.
Figure 3
Establishment of normal prostate and prostate adenocarcinoma cell cultures from the same patient. Biopsy specimens of normal and malignant prostate tissue from the same patient were processed and propagated in vitro, as described in Materials and Methods. For two-dimensional cultures, cells were photographed at days 2 and 3, demonstrating the rapid and equivalent outgrowth of cells from the biopsy specimens. For in vitro three-dimensional cultures, matched cells were also grown in the three-dimensional Matrigel system, as described in Materials and Methods. The normal prostate cells formed prostaspheres, whereas the tumor cells formed large undifferentiated aggregates. For an in vivo xenograft, 1 million normal and tumor cells were injected s.c. into the flanks of five mice with severe combined immunodeficiency at two different sites (a total of 10 sites for each cell type). The prostate cancer cells induced tumors at 7 of 10 sites within 8 weeks; a representative tumor is shown (arrow). The normal prostate cells did not induce tumors (0 of 10 sites). epi, epithelial cells.
Figure 4
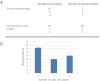
Colony-forming efficiency of primary human cells. Human keratinocytes and cervical cancer cells (Caski) were plated at the indicated cell numbers onto the feeder cell cultures. After 7 to 10 days, the feeder cells were removed and the epithelial colonies were fixed for 5 minutes with 3.7% paraformaldehyde, and then stained for 30 minutes with 0.05% crystal violet. The plates were photographed and the colonies were counted with ChemiDoc and QuantityOne software (Bio-Rad Laboratories, Inc, Hercules, CA). A: The number of cells plated was plotted versus the number of colonies observed after 7 to 10 days. B: The efficiency of colony formation for the cervical cancer cells is depicted for the indicated number of Caski cells plated. The plating efficiency for low-density seeding ranged from 40% to 70%. Data are presented as mean ± SEM.
Figure 5
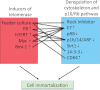
Potential model for the cooperative effects of F medium plus feeders and the ROCK inhibitor in cell immortalization. Class 1 genes induce telomerase activity, and class 2 genes alter both the p16/Rb pathway and the cell cytoskeleton. The connecting lines indicate documented interactions between class 1 and class 2 genes that result in cell immortalization.
Comment in
- Making every cell like HeLa a giant step for cell culture.
Agarwal S, Rimm DL. Agarwal S, et al. Am J Pathol. 2012 Feb;180(2):443-5. doi: 10.1016/j.ajpath.2011.12.001. Epub 2011 Dec 20. Am J Pathol. 2012. PMID: 22192626 No abstract available.
Similar articles
- Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells.
Suprynowicz FA, Upadhyay G, Krawczyk E, Kramer SC, Hebert JD, Liu X, Yuan H, Cheluvaraju C, Clapp PW, Boucher RC Jr, Kamonjoh CM, Randell SH, Schlegel R. Suprynowicz FA, et al. Proc Natl Acad Sci U S A. 2012 Dec 4;109(49):20035-40. doi: 10.1073/pnas.1213241109. Epub 2012 Nov 19. Proc Natl Acad Sci U S A. 2012. PMID: 23169653 Free PMC article. - Making every cell like HeLa a giant step for cell culture.
Agarwal S, Rimm DL. Agarwal S, et al. Am J Pathol. 2012 Feb;180(2):443-5. doi: 10.1016/j.ajpath.2011.12.001. Epub 2011 Dec 20. Am J Pathol. 2012. PMID: 22192626 No abstract available. - Conditional Reprogramming for Patient-Derived Cancer Models and Next-Generation Living Biobanks.
Palechor-Ceron N, Krawczyk E, Dakic A, Simic V, Yuan H, Blancato J, Wang W, Hubbard F, Zheng YL, Dan H, Strome S, Cullen K, Davidson B, Deeken JF, Choudhury S, Ahn PH, Agarwal S, Zhou X, Schlegel R, Furth PA, Pan CX, Liu X. Palechor-Ceron N, et al. Cells. 2019 Oct 27;8(11):1327. doi: 10.3390/cells8111327. Cells. 2019. PMID: 31717887 Free PMC article. Review. - ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells.
Li X, Krawetz R, Liu S, Meng G, Rancourt DE. Li X, et al. Hum Reprod. 2009 Mar;24(3):580-9. doi: 10.1093/humrep/den404. Epub 2008 Dec 4. Hum Reprod. 2009. PMID: 19056770 - Conditional cell reprogramming for modeling host-virus interactions and human viral diseases.
Liu X, Mondal AM. Liu X, et al. J Med Virol. 2020 Nov;92(11):2440-2452. doi: 10.1002/jmv.26093. Epub 2020 Jun 16. J Med Virol. 2020. PMID: 32478897 Free PMC article. Review.
Cited by
- Establishment and serial passage of cell cultures derived from LuCaP xenografts.
Young SR, Saar M, Santos J, Nguyen HM, Vessella RL, Peehl DM. Young SR, et al. Prostate. 2013 Sep;73(12):1251-62. doi: 10.1002/pros.22610. Epub 2013 Jun 6. Prostate. 2013. PMID: 23740600 Free PMC article. - Conditionally Reprogrammed Human Normal Airway Epithelial Cells at ALI: A Physiological Model for Emerging Viruses.
Liu X, Wu Y, Rong L. Liu X, et al. Virol Sin. 2020 Jun;35(3):280-289. doi: 10.1007/s12250-020-00244-z. Epub 2020 Jun 17. Virol Sin. 2020. PMID: 32557270 Free PMC article. Review. - Airway Progenitor Clone Formation Is Enhanced by Y-27632-Dependent Changes in the Transcriptome.
Reynolds SD, Rios C, Wesolowska-Andersen A, Zhuang Y, Pinter M, Happoldt C, Hill CL, Lallier SW, Cosgrove GP, Solomon GM, Nichols DP, Seibold MA. Reynolds SD, et al. Am J Respir Cell Mol Biol. 2016 Sep;55(3):323-36. doi: 10.1165/rcmb.2015-0274MA. Am J Respir Cell Mol Biol. 2016. PMID: 27144410 Free PMC article. - Development of an adenosquamous carcinoma histopathology - selective lung metastasis model.
Lähdeniemi IAK, Devlin JR, Nagaraj AS, Talwelkar SS, Bao J, Linnavirta N, Şeref Vujaklija C, Kiss EA, Hemmes A, Verschuren EW. Lähdeniemi IAK, et al. Biol Open. 2022 Dec 15;11(12):bio059623. doi: 10.1242/bio.059623. Epub 2022 Dec 13. Biol Open. 2022. PMID: 36355420 Free PMC article. - Precision or Personalized Medicine for Cancer Chemotherapy: Is there a Role for Herbal Medicine.
Wang Z, Liu X, Ho RL, Lam CW, Chow MS. Wang Z, et al. Molecules. 2016 Jul 7;21(7):889. doi: 10.3390/molecules21070889. Molecules. 2016. PMID: 27399658 Free PMC article. Review.
References
- Castell J.V., Gomez-Lechon M.J. Liver cell culture techniques. Methods Mol Biol. 2009;481:35–46. - PubMed
- Miki J., Rhim J.S. Prostate cell cultures as in vitro models for the study of normal stem cells and cancer stem cells. Prostate Cancer Prostatic Dis. 2008;11:32–39. - PubMed
- Van der Haegen B.A., Shay J.W. Immortalization of human mammary epithelial cells by SV40 large T-antigen involves a two step mechanism. In Vitro Cell Dev Biol. 1993;29A:180–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA106400/CA/NCI NIH HHS/United States
- R01 RR032315/RR/NCRR NIH HHS/United States
- R01 CA113477/CA/NCI NIH HHS/United States
- 5P30CA051008/CA/NCI NIH HHS/United States
- P30 CA051008/CA/NCI NIH HHS/United States
- R01 CA129003/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources