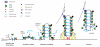Sorting nexins provide diversity for retromer-dependent trafficking events - PubMed (original) (raw)
Review
Sorting nexins provide diversity for retromer-dependent trafficking events
Peter J Cullen et al. Nat Cell Biol. 2011.
Abstract
Sorting nexins are a large family of evolutionarily conserved phosphoinositide-binding proteins that have fundamental roles in orchestrating cargo sorting through the membranous maze that is the endosomal network. One ancient group of complexes that contain sorting nexins is the retromer. Here we discuss how retromer complexes regulate endosomal sorting, and describe how this is generating exciting new insight into the central role played by endosomal sorting in development and homeostasis of normal tissues.
Figures
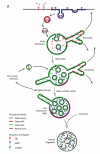
Figure 1. Sorting itineraries in the mammalian endosomal network and the distinct SNX-BAR-retromer and SNX3-retromer complexes
(A). Internalised cargo destined for degradation, such as the epidermal growth factor receptor (EGFR), are first sorted into intraluminal vesicles (ILVs) and by a process of endosomal maturation arrive in late endosomes/multivesicular bodies (MVBs). Fusion of MVBs with the lysosomal compartment brings about degradation of cargo associated with ILVs. In contrast, retrieval from the degradative pathway of, for example the nutrient sensing Transferrin receptor (TfR), occurs back to the plasma membrane via fast or slow recycling routes, while retrieval of the cation-independent mannose 6-phosphate receptor (CI-MPR) occurs back to the _trans_-Golgi network (TGN). The later route, often referred to as retrograde transport, comprises a number of distinct pathways, which include those dependent on Rab9- and SNX-BAR-retromer. Enrichment of different phosphoinositides within compartments that make up the endosomal network is shown, including the early endosomal PtdIns(3)P. (B). The distinct cargo-selective and membrane deformation sub-complexes that together form the yeast and mammalian SNX-BAR-retromers. The mammalian genome contains two VPS26’s. (C). The evolutionarily conserved mammalian SNX3-retromer complex. Gene duplication has resulted in two orthologs of C. elegans snx-3, SNX3 and SNX12.
Figure 1. Sorting itineraries in the mammalian endosomal network and the distinct SNX-BAR-retromer and SNX3-retromer complexes
(A). Internalised cargo destined for degradation, such as the epidermal growth factor receptor (EGFR), are first sorted into intraluminal vesicles (ILVs) and by a process of endosomal maturation arrive in late endosomes/multivesicular bodies (MVBs). Fusion of MVBs with the lysosomal compartment brings about degradation of cargo associated with ILVs. In contrast, retrieval from the degradative pathway of, for example the nutrient sensing Transferrin receptor (TfR), occurs back to the plasma membrane via fast or slow recycling routes, while retrieval of the cation-independent mannose 6-phosphate receptor (CI-MPR) occurs back to the _trans_-Golgi network (TGN). The later route, often referred to as retrograde transport, comprises a number of distinct pathways, which include those dependent on Rab9- and SNX-BAR-retromer. Enrichment of different phosphoinositides within compartments that make up the endosomal network is shown, including the early endosomal PtdIns(3)P. (B). The distinct cargo-selective and membrane deformation sub-complexes that together form the yeast and mammalian SNX-BAR-retromers. The mammalian genome contains two VPS26’s. (C). The evolutionarily conserved mammalian SNX3-retromer complex. Gene duplication has resulted in two orthologs of C. elegans snx-3, SNX3 and SNX12.
Figure 2. A proposed pathway for SNX-BAR-retromer-mediated sorting
Activation and cargo capture is initiated by association of the VPS26-VPS29-VPS35 sub-complex to early-to-late endosomes through interaction with the GTP-loaded form of Rab7, thus forming the nucleation complex. Not depicted are the possible roles of clathrin and various clathrin-adaptors and binding proteins in cargo clustering prior to formation of the nucleation complex. Incorporation of other cargo into the nucleation complex is achieved through specific cargo adaptors, and the SNX-BAR dimer enters the nucleation complex either by direct binding from the cytosol and/or lateral movement on the early-to-late endosomal surface. This increases the effective concentration of retromer SNX-BARs resulting in their switch to curvature inducing mode and re-model the membrane into tubular profiles. An area of concentrated VPS26-VPS29-VPS35 and SNX-BAR-binding sites is therefore formed, onto which accessory proteins are sequestered, leading to further re-modelling of the maturing tubular profile. Generation of opposing forces through actin polymerisation and motor activity aids carrier scission through either line tension and/or mechano-enzymatic fission possibly catalysed by dynamin-II and/or EHD1. Uncoating of the isolated carrier, possibly requiring ATP hydrolysis and/or Rab7-GTP hydrolysis, occurs either immediately after scission or upon tethering to the recipient compartment. See text for more details.
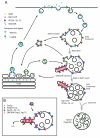
Figure 3. Spatial segregation model to account for differential cargo sorting through SNX3-retromer and SNX-BAR-retromer pathways
(A). A model based on spatial segregation to describe differential sorting of Wntless and CI-MPR by SNX3-retromer and SNX-BAR-retromer complexes respectively. Briefly, Wntless bound to Wnt morphogens is secreted to the cell surface where release of Wnt establishes short and long-range morphogenic gradients. Internalised Wntless initially enters the early endosome where it engages the SNX3-retromer for retrieval back to the TGN. In contrast, the transport of CI-MPR bound to newly synthesized hydrolase enzymes exits the TGN and is principally trafficked directly to the endosomal network, entering at a stage of maturation downstream of SNX3-retromer. The acid environment of this compartment leads to dissociation of the hydrolases, allowing retrieval of the CI-MPR via the tubular SNX-BAR-retromer. It is important to note that this model does not suggest exclusive transport of Wntless and CI-MPR through the SNX3-retromer and SNX-BAR-retromer pathway respectively. It simply reflects that steady-state transport of these cargoes appears to be dependent upon specific retromer complexes. (B). As discussed in the text, in yeast recycling of Fet3p-Ftr1p requires recognition of the cytoplasmic domain of Ftr1p by Grd19/Snx3p. Grd19/Snx3p physically associates with the SNX-BAR-retromer thereby allowing recycling of the Fet3p-Ftr1p The function of SNX3 in Wntless retrieval and Wnt secretion is therefore. fundamentally different to this role of Grd19/Snx3p.
Similar articles
- Recent advances in retromer biology.
McGough IJ, Cullen PJ. McGough IJ, et al. Traffic. 2011 Aug;12(8):963-71. doi: 10.1111/j.1600-0854.2011.01201.x. Epub 2011 May 23. Traffic. 2011. PMID: 21463457 Review. - The SNXy flavours of endosomal sorting.
Johannes L, Wunder C. Johannes L, et al. Nat Cell Biol. 2011 Jul 3;13(8):884-6. doi: 10.1038/ncb2300. Nat Cell Biol. 2011. PMID: 21725318 - Cargo selectivity of yeast sorting nexins.
Bean BD, Davey M, Conibear E. Bean BD, et al. Traffic. 2017 Feb;18(2):110-122. doi: 10.1111/tra.12459. Epub 2017 Jan 3. Traffic. 2017. PMID: 27883263 - Retromer and sorting nexins in endosomal sorting.
Gallon M, Cullen PJ. Gallon M, et al. Biochem Soc Trans. 2015 Feb;43(1):33-47. doi: 10.1042/BST20140290. Biochem Soc Trans. 2015. PMID: 25619244 Review. - The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network.
Wassmer T, Attar N, Harterink M, van Weering JR, Traer CJ, Oakley J, Goud B, Stephens DJ, Verkade P, Korswagen HC, Cullen PJ. Wassmer T, et al. Dev Cell. 2009 Jul;17(1):110-22. doi: 10.1016/j.devcel.2009.04.016. Dev Cell. 2009. PMID: 19619496 Free PMC article.
Cited by
- Snx3 regulates recycling of the transferrin receptor and iron assimilation.
Chen C, Garcia-Santos D, Ishikawa Y, Seguin A, Li L, Fegan KH, Hildick-Smith GJ, Shah DI, Cooney JD, Chen W, King MJ, Yien YY, Schultz IJ, Anderson H, Dalton AJ, Freedman ML, Kingsley PD, Palis J, Hattangadi SM, Lodish HF, Ward DM, Kaplan J, Maeda T, Ponka P, Paw BH. Chen C, et al. Cell Metab. 2013 Mar 5;17(3):343-52. doi: 10.1016/j.cmet.2013.01.013. Epub 2013 Feb 14. Cell Metab. 2013. PMID: 23416069 Free PMC article. - Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer.
Jia D, Gomez TS, Billadeau DD, Rosen MK. Jia D, et al. Mol Biol Cell. 2012 Jun;23(12):2352-61. doi: 10.1091/mbc.E11-12-1059. Epub 2012 Apr 18. Mol Biol Cell. 2012. PMID: 22513087 Free PMC article. - HPV virions hitchhike a ride on retromer complexes.
Sapp MJ. Sapp MJ. Proc Natl Acad Sci U S A. 2013 Apr 30;110(18):7116-7. doi: 10.1073/pnas.1305245110. Epub 2013 Apr 18. Proc Natl Acad Sci U S A. 2013. PMID: 23599281 Free PMC article. No abstract available. - Sorting nexins 1 and 2a locate mainly to the TGN.
Stierhof YD, Viotti C, Scheuring D, Sturm S, Robinson DG. Stierhof YD, et al. Protoplasma. 2013 Feb;250(1):235-40. doi: 10.1007/s00709-012-0399-1. Epub 2012 Mar 24. Protoplasma. 2013. PMID: 22447127 - Alleviating vacuolar transport improves cellulase production in Trichoderma reesei.
Yan S, Xu Y, Tao XM, Yu XW. Yan S, et al. Appl Microbiol Biotechnol. 2023 Apr;107(7-8):2483-2499. doi: 10.1007/s00253-023-12478-4. Epub 2023 Mar 14. Appl Microbiol Biotechnol. 2023. PMID: 36917273
References
- Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–582. - PubMed
- Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135:1175–1187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources