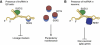Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors - PubMed (original) (raw)
Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors
Shi-Yan Ng et al. EMBO J. 2012.
Abstract
Long non-coding RNAs (lncRNAs) are a numerous class of newly discovered genes in the human genome, which have been proposed to be key regulators of biological processes, including stem cell pluripotency and neurogenesis. However, at present very little functional characterization of lncRNAs in human differentiation has been carried out. In the present study, we address this using human embryonic stem cells (hESCs) as a paradigm for pluripotency and neuronal differentiation. With a newly developed method, hESCs were robustly and efficiently differentiated into neurons, and we profiled the expression of thousands of lncRNAs using a custom-designed microarray. Some hESC-specific lncRNAs involved in pluripotency maintenance were identified, and shown to physically interact with SOX2, and PRC2 complex component, SUZ12. Using a similar approach, we identified lncRNAs required for neurogenesis. Knockdown studies indicated that loss of any of these lncRNAs blocked neurogenesis, and immunoprecipitation studies revealed physical association with REST and SUZ12. This study indicates that lncRNAs are important regulators of pluripotency and neurogenesis, and represents important evidence for an indispensable role of lncRNAs in human brain development.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
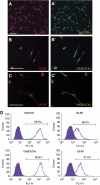
Neural progenitors derived from H1 hESCs (H1-NPCs) homogenously expressed neural stem cell and radial glia markers. (A, A′) MUSASHI-1 (MSI1) and NESTIN (NES), both neural stem cell markers, were co-expressed in almost all the NPCs. (B–C′) The elongated H1-NPCs co-expressed radial glia markers BLBP, VIMENTIN (VIM) and GFAP. The scale bar indicates 100 μm. (D) Flow cytometry quantification of lineage markers in neural progenitors. Values show the percentage of immunopositive cells for the indicated antibodies.
Figure 2
H1-NPCs differentiate into TH+ midbrain DA neurons with high efficiency. (A) MAP2-expressing mature neurons were abundant at the end of the 14-day differentiation of H1-NPCs. (B, C) These neurons also expressed TH, indicative of a dopaminergic neuronal population. (D) In three independent experiments, the mean percentages of TH+/MAP2+ cells were 85.3% and 80.4% for H1- and iPSC-derived cultures, respectively. A panel of dopaminergic markers including (E) VMAT2, (F) PITX3 and (G) DA were also expressed by the H1-derived DA neurons. (H) In an in vitro test of function, cultured H1-derived DA neurons were induced to depolarize in 56 mM KCl. Using a DA ELISA assay, DA detected in the supernatant of the 56-mM KCl treatment was significantly 100-fold higher than dopaminer detected in the CM control—medium conditioned with the DA neurons under standard culture conditions for 48 h. (I) qPCR measurement of mRNAs encoding midbrain markers in cDNA from total brain, H1-derived neural precursors and dopaminergic neurons. (J) qPCR measurement of various neuronal subtype markers: GABAergic subtype (GAD65), motor neuron subtype (ISLET1 and HB9), serotonergic neurons (TPH1 and SERT) and noradrenergic neurons (DBH), indicative of an enriched dopaminergic population. The white scale bar indicates 100 μm. * and ** indicate _P_-values of <0.05 and <0.01, respectively.
Figure 3
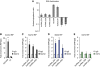
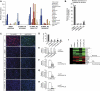
ES-specific lncRNAs are low abundance transcripts, and are probably regulated by pluripotent factors OCT4 and NANOG. (A) The expression levels of pluripotency lncRNAs were determined by qPCR in a panel of cell lines and somatic tissues. Three lncRNAs, which we named lncRNA_ES1, lncRNA_ES2 and lncRNA_ES3, were exclusively expressed in hESCs and iPSCs. (B) Comparison of lncRNA expression, as determined by qPCR, compared with the mRNA encoding OCT4. (C) Schematic showing OCT4- and NANOG-binding sites in the vicinity of the lncRNAs. Black arrows indicate transcription start sites, and thick black bars indicate exons. Binding site locations were obtained from ChIP-seq data in Chia et al (2010). (D, E) qPCR measurement of lncRNA expression changes in response to knockdown by RNAi of OCT4 and NANOG, respectively. ACTB encodes a housekeeping gene.
Figure 4
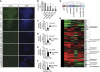
Knockdown of pluripotency lncRNAs results in hESC differentiation. (A) H1 hESCs were transfected with the indicated siRNAs, and OCT4 protein level was assayed by immunofluorescence 7 days later. (B) The percentage of OCT4+ cells from (A) was quantified by flow cytometry. (C) Assessing the efficiency of siRNA knockdown by qPCR for OCT4. (D–F) Knockdown of lncRNA_ES1, lncRNA_ES2 and lncRNA_ES3, respectively. Two siRNA duplexes were designed against each transcript, and the most effective siRNA was used. * and ** indicate _P_-values of <0.05 and <0.01, respectively. (G) Hierarchical clustering of the siRNA-treated hESCs. (H) From the microarray, a panel of pluripotency and lineage markers were analysed and fold change values, relative to si-NT control, were presented in a heatmap. Green indicates downregulation and red indicates upregulation.
Figure 5
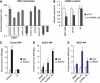
Nuclear pluripotency lncRNAs are physically associated with PRC2 component SUZ12 and pluripotent transcription factor SOX2. (A) Cellular RNA was separated into the nuclear and cytoplasmic fraction, and qPCR was performed to compute nuclear/cytoplasmic ratio. HOTAIR and KCNQ1OT1 are known, nuclear-localized RNAs, while GAPDH and ACTB are protein-coding mRNAs expected to cytoplasmically localize. (B) RIP of HOTAIR using anti-SUZ12, a previously reported interaction (Gupta et al, 2010). RIP enrichment was measured by qRT–PCR, and values were normalized to background immunoprecipitation measured by isotype IgG. (C–E) RIP of lncRNAs using anti-SUZ12, anti-SOX2 and anti-OCT4, respectively. * and ** indicate _P_-values of <0.05 and <0.01, respectively.
Figure 6
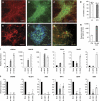
Neuronal lncRNAs are expressed in brain tissues, and are required for neurogenesis. (A) Expression of neuronal lncRNAs RMST, lncRNA_N1, lncRNA_N2 and lncRNA_N3 were analysed by qPCR in brain tissues (whole brain, fetal brain, cerebellum and substantia nigra), as well as a panel of other somatic tissues and pluripotent stem cells. RMST and lncRNA_N1 were more specific to brain tissues than lncRNA_N2 and lncRNA_N3. (B) The abundance of the neuronal lncRNAs was shown relative to GAPDH mRNA levels in H1-derived neurons. (C) Immunostaining of ReN-VM neural stem cells treated with the respective siRNAs, and induced to differentiate for 7 days. TUJ1 is a marker of early post-mitotic neurons, and MAP2 is a marker of mature neurons. (D) The percentage of TUJ1-expressing cells was quantified by flow cytometry. (E–H) We validated the efficiency of siRNAs against lncRNAs by qPCR. Shown in each case is the most efficient siRNA that was used for subsequent experiments. For RMST, all three isoforms were targeted. (I) Following knockdown and differentiation, a panel of neuronal and glia markers were analysed by qPCR, and the fold changes, relative to si-NT control, were represented in a heatmap. Green indicates downregulation and red indicates upregulation. * and ** indicate _P_-values of <0.05 and <0.01, respectively.
Figure 7
Neuronal lncRNAs act via diverse mechanisms. (A) Quantification of relative expression of lncRNAs in nuclear and cytoplasmic cell fractions. (B) Quantification of changes in hosted miRNAs in response to lncRNA_N2 knockdown. MiRNAs were quantified using Taqman miRNA qPCR. (C–E) RIP of lncRNAs with SUZ12 and REST antibodies. The interaction of HOTAIR with SUZ12 is a known interaction that serves as a positive control (Gupta et al, 2010). * and ** indicate _P_-values of <0.05 and <0.01, respectively.
Figure 8
Proposed mechanism for role of lncRNA in hESC pluripotency and neuronal differentiation. (A) In undifferentiated hESCs, we propose a mechanism whereby lncRNAs serve as a modular scaffold for SUZ12 (or PRC2) and SOX2. SOX2 may recruit the chromatin-modifying complex to silence neuroectoderm lineage markers by H3K27 methylation. Binding of lncRNA to SOX2 may prevent OCT4 or other core ES transcription factors from binding. Since lncRNAs are present in low abundance, that would not affect the core ES transcriptional network by sequestering SOX2. (B) In a mechanism similar to (A), we envisioned that neuronal lncRNAs may link chromatin modifiers and transcription factors, in order to silence glia lineage genes while promoting neurogenesis.
Comment in
- Noncoding RNA scaffolds in pluripotency.
Mondal T, Kanduri C. Mondal T, et al. Circ Res. 2012 Apr 27;110(9):1162-5. doi: 10.1161/RES.0b013e318257c489. Circ Res. 2012. PMID: 22539754 No abstract available.
Similar articles
- Loss of non-coding RNA expression from the DLK1-DIO3 imprinted locus correlates with reduced neural differentiation potential in human embryonic stem cell lines.
Mo CF, Wu FC, Tai KY, Chang WC, Chang KW, Kuo HC, Ho HN, Chen HF, Lin SP. Mo CF, et al. Stem Cell Res Ther. 2015 Jan 5;6(1):1. doi: 10.1186/scrt535. Stem Cell Res Ther. 2015. PMID: 25559585 Free PMC article. - RNA-Seq of human neurons derived from iPS cells reveals candidate long non-coding RNAs involved in neurogenesis and neuropsychiatric disorders.
Lin M, Pedrosa E, Shah A, Hrabovsky A, Maqbool S, Zheng D, Lachman HM. Lin M, et al. PLoS One. 2011;6(9):e23356. doi: 10.1371/journal.pone.0023356. Epub 2011 Sep 7. PLoS One. 2011. PMID: 21915259 Free PMC article. - lincRNAs act in the circuitry controlling pluripotency and differentiation.
Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, Yang X, Amit I, Meissner A, Regev A, Rinn JL, Root DE, Lander ES. Guttman M, et al. Nature. 2011 Aug 28;477(7364):295-300. doi: 10.1038/nature10398. Nature. 2011. PMID: 21874018 Free PMC article. - Long noncoding RNAs in cell differentiation and pluripotency.
Chen L, Zhang S. Chen L, et al. Cell Tissue Res. 2016 Dec;366(3):509-521. doi: 10.1007/s00441-016-2451-5. Epub 2016 Jun 30. Cell Tissue Res. 2016. PMID: 27365087 Review. - The Role of SMAD2/3 in Human Embryonic Stem Cells.
Yang J, Jiang W. Yang J, et al. Front Cell Dev Biol. 2020 Jul 21;8:653. doi: 10.3389/fcell.2020.00653. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850796 Free PMC article. Review.
Cited by
- Searching for convergent pathways in autism spectrum disorders: insights from human brain transcriptome studies.
Gokoolparsadh A, Sutton GJ, Charamko A, Green NF, Pardy CJ, Voineagu I. Gokoolparsadh A, et al. Cell Mol Life Sci. 2016 Dec;73(23):4517-4530. doi: 10.1007/s00018-016-2304-0. Epub 2016 Jul 12. Cell Mol Life Sci. 2016. PMID: 27405608 Free PMC article. Review. - Long noncoding RNAs and neuroblastoma.
Pandey GK, Kanduri C. Pandey GK, et al. Oncotarget. 2015 Jul 30;6(21):18265-75. doi: 10.18632/oncotarget.4251. Oncotarget. 2015. PMID: 26087192 Free PMC article. Review. - Pwp1 regulates telomere length by stabilizing shelterin complex and maintaining histone H4K20 trimethylation.
Yu Y, Jia W, Lyu Y, Su D, Bai M, Shen J, Qiao J, Han T, Liu W, Chen J, Chen W, Ye D, Guo X, Zhu S, Xi J, Zhu R, Wan X, Gao S, Zhu J, Kang J. Yu Y, et al. Cell Discov. 2019 Nov 5;5:47. doi: 10.1038/s41421-019-0116-8. eCollection 2019. Cell Discov. 2019. PMID: 31754456 Free PMC article. - Long non-coding RNAs: definitions, functions, challenges and recommendations.
Mattick JS, Amaral PP, Carninci P, Carpenter S, Chang HY, Chen LL, Chen R, Dean C, Dinger ME, Fitzgerald KA, Gingeras TR, Guttman M, Hirose T, Huarte M, Johnson R, Kanduri C, Kapranov P, Lawrence JB, Lee JT, Mendell JT, Mercer TR, Moore KJ, Nakagawa S, Rinn JL, Spector DL, Ulitsky I, Wan Y, Wilusz JE, Wu M. Mattick JS, et al. Nat Rev Mol Cell Biol. 2023 Jun;24(6):430-447. doi: 10.1038/s41580-022-00566-8. Epub 2023 Jan 3. Nat Rev Mol Cell Biol. 2023. PMID: 36596869 Free PMC article. Review. - Non-coding RNAs as epigenetic regulator of glioma stem-like cell differentiation.
Katsushima K, Kondo Y. Katsushima K, et al. Front Genet. 2014 Feb 3;5:14. doi: 10.3389/fgene.2014.00014. eCollection 2014. Front Genet. 2014. PMID: 24550934 Free PMC article. Review.
References
- Amaral PP, Mattick JS (2008) Noncoding RNA in development. Mamm Genome 19: 454–492 - PubMed
- Bellucci M, Agostini F, Masin M, Tartaglia GG (2011) Predicting protein associations with long noncoding RNAs. Nat Methods 8: 444–445 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources