Primary amines protect against retinal degeneration in mouse models of retinopathies - PubMed (original) (raw)
Primary amines protect against retinal degeneration in mouse models of retinopathies
Akiko Maeda et al. Nat Chem Biol. 2011.
Abstract
Vertebrate vision is initiated by photoisomerization of the visual pigment chromophore 11-cis-retinal and is maintained by continuous regeneration of this retinoid through a series of reactions termed the retinoid cycle. However, toxic side reaction products, especially those involving reactive aldehyde groups of the photoisomerized product, all-trans-retinal, can cause severe retinal pathology. Here we lowered peak concentrations of free all-trans-retinal with primary amine-containing Food and Drug Administration (FDA)-approved drugs that did not inhibit chromophore regeneration in mouse models of retinal degeneration. Schiff base adducts between all-trans-retinal and these amines were identified by MS. Adducts were observed in mouse eyes only when an experimental drug protected the retina from degeneration in both short-term and long-term treatment experiments. This study demonstrates a molecular basis of all-trans-retinal-induced retinal pathology and identifies an assemblage of FDA-approved compounds with protective effects against this pathology in a mouse model that shows features of Stargardt's disease and age-related retinal degeneration.
Conflict of interest statement
Competing financial interests Case Western Reserve University, and Visum Inc. may commercialize some of the technology described in this work. AM, MG and TM are consultants for Visum Inc. and KP and WH are co-founders of Visum Inc.
Figures
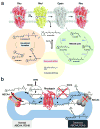
Figure 1. Retinoid cycle and fate of atRAL in the retina
(a) The visual chromophore, 11-_cis_-retinal, is photoisomerized to atRAL and released after photoactivation. Possible fates of the released atRAL are to: (1) enter the retinoid cycle for recycling, (2) form bis-retinoids, and/or (3) persist as a free toxic aldehyde that directly impairs cell survival. Most of the atRAL is used to regenerate 11-_cis_-retinal through the retinoid cycle. But a fraction of atRAL forms bis-retinoid conjugate products such as A2E and atRAL dimer that eventually accumulate and contribute to damaging the RPE. Over-production of atRAL can induce retinal degeneration due to intense light illumination and genetic defects in RDH8 and ABCA4, which are critical for atRAL processing. These observations form the basis of our hypothesis that treatment with primary amines that form a Schiff base will lower excessive amounts of atRAL produced in mice by aging or lack of genes encoding ABCA4 and RDH8. (b) The biological role of ABCA4 and RDH8 in processing atRAL in ROS is illustrated. The left side of the diagram represents a ROS disk with a functional ABCA4 transporter and RDH8, whereas the right side portrays a ROS disk with an inactivated ABCA4 and RDH8. In rods, photoactivated rhodopsin releases atRAL outside (cytoplasm) and inside the disc lumen. Released atRAL in the disc lumen then reacts with PE producing _N_-retinylidene phosphatidylethanolamine (_N_-ret-PE). _N_-ret-PE is transported to the cytoplasm and releases atRAL that leaves the disc where it is converted to atROL.
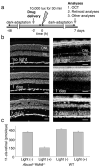
Figure 2. Testing effects of amines on the development of acute light-induced retinal degeneration in Abca−/−Rdh8−/− mice
(a) Schematic representation of the experimental design.Four-week-old Abca4−/−Rdh8−/− mice were kept in the dark for 48 h when a single dose of drug was administered by oral gavage 2 h before light exposure at 10,000 lux for 30 min. Mice then were kept in the dark for 7 days when final retina evaluations were performed. (b) Four-week-old _Abca4−/−Rdh8−/−_mice evidenced severe retinal degeneration 7 days after bright light exposure whereas Abca4−/−Rdh8−/− mice maintained under regular laboratory lighting did not. Cryosections (left column) reveal retinal damage 7 days after bright light exposure whereas OCT images (right column) show hazy changes in the outer nuclear layer (ONL) one day after light exposure and reduced thickness of the ONL 7 days after light exposure. INL, inner nuclear layer. Bars indicate 20 μm in cryo-sections and 50 μm in OCT. (c) Amounts of 11-_cis_-retinal in the eye reflecting photoreceptor number were quantified by HPLC 7 days after 4-week-old Abca4−/−Rdh8−/− mice were exposed to 10,000 lux for 30 min. Light-illuminated Abca4−/−Rdh8−/− mice showed reduced amounts of 11-_cis_-retinal whereas 4-week-old WT mice did not.
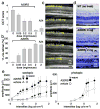
Figure 3. Effects of pre-administered amine drugs on light-induced acute retinal degeneration in 4-week-old Abca4−/−Rdh8−/− mice
(a) Pretreatment with A20RS protected the retina from light induced damage in a dose-dependent manner as assessed by OCT, with 2 mg/mouse completely preserving retinal morphology. Included as a positive control, A24 (2 mg/mouse) also protected the retina . Experiments were performed with the protocol shown in Fig. 2a. (b) Amounts of 11-_cis_-retinal in the eye also were maintained in a dose-dependent manner by A20RS pretreatment, an effect replicated by pretreatment with A24 (2 mg/mouse). (c) Shown are representative OCT images from light-illuminated 4-week-old Abca4−/−Rdh8−/− mice pretreated with various doses of A20RS. Retinal morphology was preserved in a dose-dependent manner. INL, inner nuclear layer; ONL, outer nuclear layer. (d) Representative Epon-prepared retinal sections from light-illuminated 4-week-old Abca4−/−Rdh8−/− and WT mice are shown. Retinal histology was completely preserved by pretreatment with A20RS (2 mg/mouse). RPE, retinal pigmented epithelium; PR, photoreceptors; ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer. Bars indicate 20 μm in cryo-sections and 50 μm in OCT. (e) Full field ERG responses of A20RS- or vehicle-pretreated light-illuminated Abca4−/−Rdh8−/− mice at 4 weeks of age. ERG responses were recorded under scotopic (left) and photopic (right) conditions. Both scotopic and photopic ERG amplitudes plotted as a function of light intensity were better preserved in A20RS-pretreated Abca4−/−Rdh8−/− mice as compared with vehicle-pretreated animals. Bars indicate S.D. of the means (n > 3).
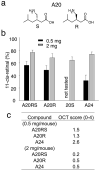
Figure 4. A20 stereoisomers protect against light-induced acute retinal degeneration in Abca4−/−Rdh8−/− mice
(a) Enantiomers of A20 are shown (R and S). The S-isomer of A20 is pregabalin, an analgesic that affects Ca2+ channels. (b) Amounts of 11-_cis_-retinal reflecting photoreceptor number were quantified by HPLC in the eyes of 4-week-old Abca4−/−Rdh8−/− mice 7 days after exposure to light at 10,000 lux for 30 min. Synthesized stereoisomers of A20 (A20RS and A20R), and a drug formulation A20S at 0.5 and 2 mg/mouse were orally gavaged 2 h before bright light exposure. All isomers of A20 showed similar protective effects against light-induced retinal degeneration. A24 was used as a positive control. Error bars indicate S.D. of the means (n > 3). (c) In vivo imaging by OCT was performed as described in Supplementary Fig. 1. OCT Grades for 0.5 and 2 mg of stereoisomer-treated mice are shown.
Figure 5. Identification of retinal conjugates in retinas of Abca4−/−Rdh8−/− mice treated with A2 and A20
These mice were gavaged with test amines 2 h before exposure to intense light at 10,000 lux for 20 min; one hour later they were euthanized and retinoids extracted from whole eyeballs were analyzed. (a) Chromatographic separation of synthetic standards represented by selected ion chromatograms for A2 (gray) and A20 (black) retinyl imines (at m/z = 446.5 [MH]+ and m/z = 426.5 [MH]+, respectively). Panel b shows corresponding UV/Vis spectra of their protonated Schiff bases. (c) Sample extracted from mice treated with A4. Extracted ion chromatogram at m/z = 446.5 indicates a peak with an elution time, molecular mass (d) and ion fragmentation pattern (d, inset) that is identical with that of the synthetic standard A2 retinyl imine. Importantly, samples extracted from control untreated mice lacked a distinct peak at m/z = 446.5. Panels e and f represent an analogous set of data for samples extracted from the eyes of mice treated with A20. Thus, oral administration of these tested primary amines led to formation of their corresponding Schiff bases detected in organic extracts of the eyes.
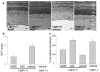
Figure 6. Effects of treatment with amine drugs on age-related retinal degeneration in Abca4−/−Rdh8−/− mice
Abca4−/−Rdh8−/− mice were gavaged daily with either A20RS (2 mg/mouse) or A22 (2 mg/mouse) and weekly with A24 (2 mg/mouse) for 3 months beginning at 1 month of age. (a) Representative SLO (upper) and OCT (lower) images of A24-pretreated Abca4−/−Rdh8−/− mice are shown. INL, inner nuclear layer; ONL, outer nuclear layer. A24 prevented both an increase of fundus autofluorescence intensity and retinal degeneration. Bars indicate 20 μm in cryo-sections and 50 μm in OCT. (b) Amounts of A2E were quantified by reverse phase HPLC after 3 months of treatment of Abca4−/−Rdh8−/− mice by oral gavage. A20RS, A22, and A24 treatment reduced accumulation of A2E relative to vehicle-treated animals. Error bars indicate S.D. of the means (n = 3 for A20RS, 3 for A22, 3 for A24 and 4 for vehicle). (c) OCT analyses of Abca4−/−Rdh8−/− mouse retinas performed 3 months after treatment of one-month-old animals with either A20RS, A22 or A24 reveal maintenance of retinal structure. Error bars indicate S.D. of the means (n = 15 for A20RS, 15 for A22, 10 for A24 and 22 for vehicle). (d) Morphology of retinas from 4-month-old Abca4−/−Rdh8−/− mice treated with A20RS, A22, A24 or vehicle for 3 months. Retinas from drug-treated mice evidenced preserved structures compared with vehicle-pretreated animals. RPE, retinal pigmented epithelium; OS, outer segment; IS, inner segment; ONL, outer nuclear layer. Bars indicate 20 μm.
Figure 7. Effects of pre-administered amine drugs on light-induced retinal degeneration in BALB/c mice
(a) Representative epon-prepared retinal sections from light-illuminated 6-week-old WT (BALB/c) mice are shown. Mice were illuminated with 5,000 lux for 2 h, and analysis was performed 7 days later. Pretreatment with A20R (2 mg/mouse, 2 h before light) partially protected the retina from light induced damage, and A24, included as a positive control (2 mg/mouse, 16 h before light) protected the retina as previously reported . RPE, retinal pigmented epithelium; OS, outer segment; IS, inner segment; ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Bars indicate 10 μm. (b) OCT analyses of WT mouse retinas were performed. Score 0, no degeneration; score 1, reduced ONL thickness with more than 50% of ONL remaining as compared to no-illuminated animals; score 2, reduced ONL thickness with less than 50% of ONL for non-illuminated animals; score 3, severe degeneration without reflection from the ONL layer. A20R provided partial protection of retinal structure, whereas A24 protected retinal degeneration completely. Error bars indicate S.D. of the means (n = 12 for A20R, 7 for A24 and 15 for vehicle). (c) Amounts of 11-_cis_-retinal in the eye quantified by HPLC also were partially preserved by A20R (2 mg/mouse) and completely protected by A24 pretreatment. Bars indicate S.D. of the means (n > 3).
Similar articles
- The novel visual cycle inhibitor (±)-RPE65-61 protects retinal photoreceptors from light-induced degeneration.
Wang Y, Ma X, Muthuraman P, Raja A, Jayaraman A, Petrukhin K, Cioffi CL, Ma JX, Moiseyev G. Wang Y, et al. PLoS One. 2022 Oct 13;17(10):e0269437. doi: 10.1371/journal.pone.0269437. eCollection 2022. PLoS One. 2022. PMID: 36227868 Free PMC article. - Protective Effect of a Locked Retinal Chromophore Analog against Light-Induced Retinal Degeneration.
Gao S, Parmar T, Palczewska G, Dong Z, Golczak M, Palczewski K, Jastrzebska B. Gao S, et al. Mol Pharmacol. 2018 Oct;94(4):1132-1144. doi: 10.1124/mol.118.112581. Epub 2018 Jul 17. Mol Pharmacol. 2018. PMID: 30018116 Free PMC article. - Molecular pharmacodynamics of emixustat in protection against retinal degeneration.
Zhang J, Kiser PD, Badiee M, Palczewska G, Dong Z, Golczak M, Tochtrop GP, Palczewski K. Zhang J, et al. J Clin Invest. 2015 Jul 1;125(7):2781-94. doi: 10.1172/JCI80950. Epub 2015 Jun 15. J Clin Invest. 2015. PMID: 26075817 Free PMC article. - Vitamin A and Vision.
Saari JC. Saari JC. Subcell Biochem. 2016;81:231-259. doi: 10.1007/978-94-024-0945-1_9. Subcell Biochem. 2016. PMID: 27830507 Review. - Key enzymes of the retinoid (visual) cycle in vertebrate retina.
Kiser PD, Golczak M, Maeda A, Palczewski K. Kiser PD, et al. Biochim Biophys Acta. 2012 Jan;1821(1):137-51. doi: 10.1016/j.bbalip.2011.03.005. Epub 2011 Apr 5. Biochim Biophys Acta. 2012. PMID: 21447403 Free PMC article. Review.
Cited by
- Scavenging of Cation Radicals of the Visual Cycle Retinoids by Lutein, Zeaxanthin, Taurine, and Melanin.
Rozanowska M, Edge R, Land EJ, Navaratnam S, Sarna T, Truscott TG. Rozanowska M, et al. Int J Mol Sci. 2023 Dec 29;25(1):506. doi: 10.3390/ijms25010506. Int J Mol Sci. 2023. PMID: 38203675 Free PMC article. - Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration.
Chen Y, Okano K, Maeda T, Chauhan V, Golczak M, Maeda A, Palczewski K. Chen Y, et al. J Biol Chem. 2012 Feb 10;287(7):5059-69. doi: 10.1074/jbc.M111.315432. Epub 2011 Dec 19. J Biol Chem. 2012. PMID: 22184108 Free PMC article. - CCL3 production by microglial cells modulates disease severity in murine models of retinal degeneration.
Kohno H, Maeda T, Perusek L, Pearlman E, Maeda A. Kohno H, et al. J Immunol. 2014 Apr 15;192(8):3816-27. doi: 10.4049/jimmunol.1301738. Epub 2014 Mar 17. J Immunol. 2014. PMID: 24639355 Free PMC article. - Pharmacological inhibition of lipofuscin accumulation in the retina as a therapeutic strategy for dry AMD treatment.
Petrukhin K. Petrukhin K. Drug Discov Today Ther Strateg. 2013;10(1):e11-e20. doi: 10.1016/j.ddstr.2013.05.004. Drug Discov Today Ther Strateg. 2013. PMID: 25152755 Free PMC article. - Multifunctional PEG retinylamine conjugate provides prolonged protection against retinal degeneration in mice.
Yu G, Wu X, Ayat N, Maeda A, Gao SQ, Golczak M, Palczewski K, Lu ZR. Yu G, et al. Biomacromolecules. 2014 Dec 8;15(12):4570-8. doi: 10.1021/bm501352s. Epub 2014 Nov 20. Biomacromolecules. 2014. PMID: 25390360 Free PMC article.
References
- Palczewski K, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. - PubMed
- Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275:11034–11043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 EY019031/EY/NEI NIH HHS/United States
- R01 EY009339/EY/NEI NIH HHS/United States
- P30 EY011373/EY/NEI NIH HHS/United States
- EY019880/EY/NEI NIH HHS/United States
- K08 EY019031-03/EY/NEI NIH HHS/United States
- R24 EY021126-02/EY/NEI NIH HHS/United States
- K08 EY019880/EY/NEI NIH HHS/United States
- R01 EY009339-22/EY/NEI NIH HHS/United States
- R24 EY021126/EY/NEI NIH HHS/United States
- EY009339/EY/NEI NIH HHS/United States
- EY021126/EY/NEI NIH HHS/United States
- P30 EY11373/EY/NEI NIH HHS/United States
- K08 EY019031-04/EY/NEI NIH HHS/United States
- EY019031/EY/NEI NIH HHS/United States
- K08 EY019880-03/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases