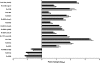Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions - PubMed (original) (raw)
Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions
Jamie Harper et al. J Infect Dis. 2012.
Abstract
Background: Preclinical evaluation of tuberculosis drugs is generally limited to mice. However, necrosis and hypoxia, key features of human tuberculosis lesions, are lacking in conventional mouse strains.
Methods: We used C3HeB/FeJ mice, which develop necrotic lesions in response to Mycobacterium tuberculosis infection. Positron emission tomography in live infected animals, postmortem pimonidazole immunohistochemistry, and bacterial gene expression analyses were used to assess whether tuberculosis lesions in C3HeB/FeJ are hypoxic. Efficacy of combination drug treatment, including PA-824, active against M. tuberculosis under hypoxic conditions, was also evaluated.
Results: Tuberculosis lesions in C3HeB/FeJ (but not BALB/c) were found to be hypoxic and associated with up-regulation of known hypoxia-associated bacterial genes (P < .001). Contrary to sustained activity reported elsewhere in BALB/c mice, moxifloxacin and pyrazinamide (MZ) combination was not bactericidal beyond 3 weeks in C3HeB/FeJ. Although PA-824 added significant activity, the novel combination of PA-824 and MZ was less effective than the standard first-line regimen in C3HeB/FeJ.
Conclusions: We demonstrate that tuberculosis lesions in C3HeB/FeJ are hypoxic. Activities of some key tuberculosis drug regimens in development are represented differently in C3HeB/FeJ versus BALB/c mice. Because C3HeB/FeJ display key features of human tuberculosis, this strain warrants evaluation as a more pathologically relevant model for preclinical studies.
Figures
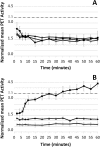
Figure 1.
Positron emission tomographic (PET) imaging demonstrates accumulation of hypoxia probe copper(II)-diacetyl-bis(_N_4-methyl-thiosemicarbazone) ([64Cu]ATSM) in tuberculosis lesions of C3HeB/FeJ mice. The mean [64Cu]ATSM PET lung activity normalized to the thigh muscles from dynamic acquisitions is shown for acute (A) and chronic infection (B) time points. During the chronic phase, progressive time-dependent accumulation of [64Cu]ATSM was observed in pulmonary tuberculosis lesions of infected C3HeB/FeJ mice (squares), with no accumulation observed in the infected BALB/c (triangles) and uninfected C3HeB/FeJ (X's) mice (P < .001). No [64Cu]ATSM accumulation was noted during the acute phase. Data are presented as means ± standard deviations.
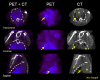
Figure 2.
Copper(II)-diacetyl-bis(_N_4-methyl-thiosemicarbazone) ([64Cu]ATSM) is localized to tuberculosis lesions of C3HeB/FeJ mice. Transverse, coronal, and sagittal computed tomographic (CT) and positron emission tomographic (PET) images from a _Mycobacterium tuberculosis_–infected C3HeB/FeJ mouse lung during the chronic phase of infection are shown. In the right panel (CT), the tuberculosis lesion is seen as a consolidation (gra y) just posterior to the heart (H) (arrows). The middle panel (PET) shows the corresponding [64Cu]ATSM PET images. The arrows point to areas of high [64Cu]ATSM PET activity and the region of interest (encircled). The left panel (PET plus CT) shows the colocalization of the [64Cu]ATSM PET signal and the tuberculosis lesion seen on the CT images.
Figure 3.
Postmortem histopathology and pimonidazole immunohistochemical analyses. Hematoxylin-eosin histology (A, C, E, G, I) and pimonidazole immunohistochemistry (B, D, F, H, J) were performed on lung sections from chronically infected C3HeB/FeJ mice 14 weeks after infection (A_–_D), BALB/c mice 8 weeks after infection (E, F), and uninfected C3HeB/FeJ mice (G, H) and on renal tubular cells in kidney tissues from C3HeB/FeJ mice (I, J). Pimonidazole staining is noted around the periphery of the necrotic granulomas (B, D). Although there is no evidence of necrosis, small foci of pimonidazole staining is observed in the chronically infected BALB/c mice (F, inset). No pimonidazole staining is observed in the uninfected C3HeB/FeJ mice (H), whereas significant pimonidazole staining is noted in the renal tubular cells in the kidney tissues (J).
Figure 4.
Quantitative real-time polymerase chain reaction (RT-PCR) for selected hypoxia-associated Mycobacterium tuberculosis genes. The bacterial transcriptional response for 14 hypoxia-associated and 3 hypoxia-independent genes (Rv0006, Rv0014c, and Rv0058) was measured by RT-PCR. M. tuberculosis isolated from the lungs of the chronically infected C3HeB/FeJ mice were compared with bacteria grown in vitro. Data are normalized to either sigA (open bars) or 16s ribosomal RNA (rRNA) (closed bars) and presented on a logarithmic scale as means ± standard deviations.
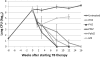
Figure 5.
Efficacy of combination tuberculosis drug treatment in C3HeB/FeJ mice. Eight weeks after a low-dose aerosol infection, C3HeB/FeJ mice were allocated to different treatment groups. Lungs from mice treated with the PMZ and PHZ regimens were culture negative after 8 weeks of treatment, whereas lungs from mice treated with the standard RHZ regimen were culture negative only after 12 weeks of treatment. After displaying significant bactericidal activity during the first 3 weeks, MZ was ineffective and even permitted bacterial multiplication. Only 5 of 6 mice treated with PaMZ were culture negative after 16 weeks of treatment. At least 4 mice were killed for each group and time point assessed. Data are presented on a logarithmic scale as means ± standard deviations. CFUs, colony-forming units; H, isoniazid; M, moxifloxicin; P, rifapentine; Pa, PA-824; R, rifampin; Z, pyrazinamide.
Similar articles
- Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis.
Lanoix JP, Lenaerts AJ, Nuermberger EL. Lanoix JP, et al. Dis Model Mech. 2015 Jun;8(6):603-10. doi: 10.1242/dmm.019513. Epub 2015 Mar 30. Dis Model Mech. 2015. PMID: 26035868 Free PMC article. - Sterilizing Activity of Pyrazinamide in Combination with First-Line Drugs in a C3HeB/FeJ Mouse Model of Tuberculosis.
Lanoix JP, Betoudji F, Nuermberger E. Lanoix JP, et al. Antimicrob Agents Chemother. 2015 Dec 7;60(2):1091-6. doi: 10.1128/AAC.02637-15. Print 2016 Feb. Antimicrob Agents Chemother. 2015. PMID: 26643352 Free PMC article. - Bedaquiline and Pyrazinamide Treatment Responses Are Affected by Pulmonary Lesion Heterogeneity in Mycobacterium tuberculosis Infected C3HeB/FeJ Mice.
Irwin SM, Prideaux B, Lyon ER, Zimmerman MD, Brooks EJ, Schrupp CA, Chen C, Reichlen MJ, Asay BC, Voskuil MI, Nuermberger EL, Andries K, Lyons MA, Dartois V, Lenaerts AJ. Irwin SM, et al. ACS Infect Dis. 2016 Apr 8;2(4):251-267. doi: 10.1021/acsinfecdis.5b00127. Epub 2016 Feb 24. ACS Infect Dis. 2016. PMID: 27227164 Free PMC article. - PA-824 , moxifloxacin and pyrazinamide combination therapy for tuberculosis.
Dawson R, Diacon A. Dawson R, et al. Expert Opin Investig Drugs. 2013 Jul;22(7):927-32. doi: 10.1517/13543784.2013.801958. Epub 2013 May 21. Expert Opin Investig Drugs. 2013. PMID: 23687915 Review. - Fighting tuberculosis by drugs targeting nonreplicating Mycobacterium tuberculosis bacilli.
Iacobino A, Piccaro G, Giannoni F, Mustazzolu A, Fattorini L. Iacobino A, et al. Int J Mycobacteriol. 2017 Jul-Sep;6(3):213-221. doi: 10.4103/ijmy.ijmy_85_17. Int J Mycobacteriol. 2017. PMID: 28776518 Review.
Cited by
- Analysis of the contribution of MTP and the predicted Flp pilus genes to Mycobacterium tuberculosis pathogenesis.
Mann KM, Pride AC, Flentie K, Kimmey JM, Weiss LA, Stallings CL. Mann KM, et al. Microbiology (Reading). 2016 Oct;162(10):1784-1796. doi: 10.1099/mic.0.000368. Epub 2016 Aug 31. Microbiology (Reading). 2016. PMID: 27586540 Free PMC article. - Transcriptional regulators SP110 and SP140 modulate inflammatory response genes in _Mycobacterium tuberculosis_-infected human macrophages.
Nakamura H, Hikichi H, Seto S, Hijikata M, Keicho N. Nakamura H, et al. Microbiol Spectr. 2024 Oct 3;12(10):e0010124. doi: 10.1128/spectrum.00101-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162523 Free PMC article. - Treatment-Shortening Effect of a Novel Regimen Combining Clofazimine and High-Dose Rifapentine in Pathologically Distinct Mouse Models of Tuberculosis.
Saini V, Ammerman NC, Chang YS, Tasneen R, Chaisson RE, Jain S, Nuermberger E, Grosset JH. Saini V, et al. Antimicrob Agents Chemother. 2019 May 24;63(6):e00388-19. doi: 10.1128/AAC.00388-19. Print 2019 Jun. Antimicrob Agents Chemother. 2019. PMID: 30936097 Free PMC article. - Cavitary tuberculosis: the gateway of disease transmission.
Urbanowski ME, Ordonez AA, Ruiz-Bedoya CA, Jain SK, Bishai WR. Urbanowski ME, et al. Lancet Infect Dis. 2020 Jun;20(6):e117-e128. doi: 10.1016/S1473-3099(20)30148-1. Epub 2020 May 5. Lancet Infect Dis. 2020. PMID: 32482293 Free PMC article. Review. - Differential Mycobacterium bovis BCG vaccine-derived efficacy in C3Heb/FeJ and C3H/HeOuJ mice exposed to a clinical strain of Mycobacterium tuberculosis.
Henao-Tamayo M, Obregón-Henao A, Creissen E, Shanley C, Orme I, Ordway DJ. Henao-Tamayo M, et al. Clin Vaccine Immunol. 2015 Jan;22(1):91-8. doi: 10.1128/CVI.00466-14. Epub 2014 Nov 12. Clin Vaccine Immunol. 2015. PMID: 25392011 Free PMC article.
References
- Nuermberger E. Using animal models to develop new treatments for tuberculosis. Semin Respir Crit Care Med. 2008;29:542–51. - PubMed
- Aly S, Wagner K, Keller C, et al. Oxygen status of lung granulomas in Mycobacterium tuberculosis–infected mice. J Pathol. 2006;210:298–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical