Vesicular and plasma membrane transporters for neurotransmitters - PubMed (original) (raw)
Review
Vesicular and plasma membrane transporters for neurotransmitters
Randy D Blakely et al. Cold Spring Harb Perspect Biol. 2012.
Abstract
The regulated exocytosis that mediates chemical signaling at synapses requires mechanisms to coordinate the immediate response to stimulation with the recycling needed to sustain release. Two general classes of transporter contribute to release, one located on synaptic vesicles that loads them with transmitter, and a second at the plasma membrane that both terminates signaling and serves to recycle transmitter for subsequent rounds of release. Originally identified as the target of psychoactive drugs, these transport systems have important roles in transmitter release, but we are only beginning to understand their contribution to synaptic transmission, plasticity, behavior, and disease. Recent work has started to provide a structural basis for their activity, to characterize their trafficking and potential for regulation. The results indicate that far from the passive target of psychoactive drugs, neurotransmitter transporters undergo regulation that contributes to synaptic plasticity.
Figures
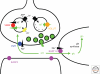
Figure 1.
Role of plasma membrane and vesicular neurotransmitter transporters in synaptic transmission. After the exocytotic release from synaptic vesicles, neurotransmitter is transported back into the terminal by Na+ and Cl−-dependent plasma membrane transporters (PMT), thereby regenerating the vesicular pools required to sustain release. In the case of glutamate, excitatory amino acid transporters (EAATs) are generally found on cells other than those directly involved in glutamate release; most of the uptake occurs into astrocytes, mediated by EAAT1 and 2, which do not couple stoichiometrically to the flux of Cl−. Nonetheless, other isoforms such as EAAT3 can be expressed by neurons, although generally not at presynaptic sites or not by glutamate neurons. The glutamate taken up by glia undergoes conversion to glutamine and is then thought to recycle to neurons through the system N transporters expressed by glia and the system A transporters expressed by neurons, with conversion back to glutamate by phosphate-activated glutaminase (PAG) within neurons. Synaptic vesicles fill with neurotransmitter through a process driven by the vacuolar-type H+-ATPase. However, different transmitters depend on different components of the H+ electrochemical gradient produced by this pump. The vesicular monoamine transporter (VMAT) and closely related vesicular acetylcholine transporter depend primarily on the chemical component, ΔpH, whereas vesicular glutamate transporters (VGLUTs) depend predominantly on the membrane potential, Δψ. The entry of anions such as Cl− (but also glutamate) promote the formation of ΔpH by dissipating Δψ and hence allowing the H+ pump to generate ΔpH, although the factors that promote Δψ have remained unexplored.
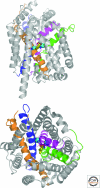
Figure 2.
Crystal structure of Gltph (PDB ID2NWX), a prokaryotic member of the SLC1 family of neurotransmitter transporters. Ribbon diagram of the Gltph trimer viewed in the membrane plane (upper panel) and from the extracellular surface (lower panel), with the protomers colored cyan, magenta, and green. L-aspartate is shown as a stick model with carbon, nitrogen, and oxygen atoms colored yellow, blue, and red, respectively. The two sodium ions identified in the structure are depicted as blue spheres. The substrates are bound at an occluded site located halfway across the membrane bilayer, near HP2, TM8, and the unwound region of TM7. (Figure courtesy of Dr. Satinder K. Singh, Department of Cellular and Molecular Physiology, Yale University School of Medicine.)
Figure 3.
Crystal structure of LeuT (PDB ID 2A65), a prokaryotic member of the SLC6 family of neurotransmitter transporters. Ribbon diagram of LeuT viewed in the plane of the membrane (upper panel) and from the extracellular surface (lower panel). TMs 1,3,6,8 are colored magenta, orange, green, and blue, respectively. L-leucine is shown as a stick model with carbon, nitrogen, and oxygen atoms colored yellow, blue, and red, respectively. The two sodium ions are depicted as cyan spheres. The substrates are bound at an occluded site at the center of the membrane bilayer, near TMs 3 and 8 and the unwound sections of TMs 1 and 6. (Figure courtesy of Dr. Satinder K. Singh, Department of Cellular and Molecular Physiology, Yale University School of Medicine.)
Similar articles
- Trafficking of vesicular neurotransmitter transporters.
Fei H, Grygoruk A, Brooks ES, Chen A, Krantz DE. Fei H, et al. Traffic. 2008 Sep;9(9):1425-36. doi: 10.1111/j.1600-0854.2008.00771.x. Epub 2008 May 26. Traffic. 2008. PMID: 18507811 Free PMC article. Review. - The synaptic vesicle cycle.
Sudhof TC. Sudhof TC. Annu Rev Neurosci. 2004;27:509-47. doi: 10.1146/annurev.neuro.26.041002.131412. Annu Rev Neurosci. 2004. PMID: 15217342 Review. - Plasma membrane GABA transporters reside on distinct vesicles and undergo rapid regulated recycling.
Deken SL, Wang D, Quick MW. Deken SL, et al. J Neurosci. 2003 Mar 1;23(5):1563-8. doi: 10.1523/JNEUROSCI.23-05-01563.2003. J Neurosci. 2003. PMID: 12629157 Free PMC article. - The neurotransmitter cycle and quantal size.
Edwards RH. Edwards RH. Neuron. 2007 Sep 20;55(6):835-58. doi: 10.1016/j.neuron.2007.09.001. Neuron. 2007. PMID: 17880890 Review. - Synaptic vesicles: half full or half empty?
Hnasko TS, Edwards RH. Hnasko TS, et al. Neuron. 2006 Sep 7;51(5):523-4. doi: 10.1016/j.neuron.2006.08.019. Neuron. 2006. PMID: 16950150 Review.
Cited by
- Transport and inhibition mechanisms of human VMAT2.
Wu D, Chen Q, Yu Z, Huang B, Zhao J, Wang Y, Su J, Zhou F, Yan R, Li N, Zhao Y, Jiang D. Wu D, et al. Nature. 2024 Feb;626(7998):427-434. doi: 10.1038/s41586-023-06926-4. Epub 2023 Dec 11. Nature. 2024. PMID: 38081299 - Wiring and Volume Transmission: An Overview of the Dual Modality for Serotonin Neurotransmission.
Gianni G, Pasqualetti M. Gianni G, et al. ACS Chem Neurosci. 2023 Dec 6;14(23):4093-4104. doi: 10.1021/acschemneuro.3c00648. Epub 2023 Nov 15. ACS Chem Neurosci. 2023. PMID: 37966717 Free PMC article. Review. - Microscopic Characterization of Membrane Transporter Function by In Silico Modeling and Simulation.
Vermaas JV, Trebesch N, Mayne CG, Thangapandian S, Shekhar M, Mahinthichaichan P, Baylon JL, Jiang T, Wang Y, Muller MP, Shinn E, Zhao Z, Wen PC, Tajkhorshid E. Vermaas JV, et al. Methods Enzymol. 2016;578:373-428. doi: 10.1016/bs.mie.2016.05.042. Epub 2016 Jul 11. Methods Enzymol. 2016. PMID: 27497175 Free PMC article. Review. - The development of synaptic transmission is time-locked to early social behaviors in rats.
Naskar S, Narducci R, Balzani E, Cwetsch AW, Tucci V, Cancedda L. Naskar S, et al. Nat Commun. 2019 Mar 13;10(1):1195. doi: 10.1038/s41467-019-09156-3. Nat Commun. 2019. PMID: 30867422 Free PMC article. - 5-HT1B receptor modulation of the serotonin transporter in vivo: studies using KO mice.
Montañez S, Munn JL, Owens WA, Horton RE, Daws LC. Montañez S, et al. Neurochem Int. 2014 Jul;73:127-31. doi: 10.1016/j.neuint.2013.11.004. Epub 2013 Nov 15. Neurochem Int. 2014. PMID: 24246466 Free PMC article.
References
- Accardi A, Miller C 2004. Secondary active transport mediated by a prokaryotic homologue of ClC Cl-channels. Nature 427: 803–807 - PubMed
- Aihara Y, Mashima H, Onda H, Hisano S, Kasuya H, Hori T, Yamada S, Tomura H, Yamada Y, Inoue I, et al. 2000. Molecular cloning of a novel brain-type Na+-dependent inorganic phosphate cotransporter. J Neurochem 74: 2622–2625 - PubMed
- Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A 2007. Glutamine in the central nervous system: Function and dysfunction. Front Biosci 12: 332–343 - PubMed
- Alfonso A, Grundahl K, Duerr JS, Han H-P, Rand JB 1993. The Caenorhabditis elegans unc-17 gene: A putative vesicular acetylcholine transporter. Science 261: 617–619 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources