Basket cell dichotomy in microcircuit function - PubMed (original) (raw)
Review
Basket cell dichotomy in microcircuit function
Caren Armstrong et al. J Physiol. 2012.
Abstract
A diversity of GABAergic cell types exist within each brain area, and each cell type is thought to play a unique role in the modulation of principal cell output. Basket cells, whose axon terminals surround principal cell somata and proximal dendrites, have a privileged and influential position for regulating the firing of principal cells. This review explores the dichotomy of the two basket cell classes, cholecystokinin- (CCK) and parvalbumin (PV)-containing basket cells, beginning with differences at the level of the individual cell and subsequently focusing on two ways in which this intrinsic dichotomy is enhanced by extrinsic factors. Neuromodulatory influences, exemplified by the effects of the peptide CCK, dynamically enhance the differential functions of the two cell types. Specifications at the level of the postsynaptic principal cell, including input-specific differences in chloride handling and differences in long-range projection patterns of the principal cell targets, also enhance the distinct network function of basket cells. In this review, new findings will be highlighted concerning the roles of neuromodulatory control and postsynaptic long-range projection pattern in the definition of basket cell function.
Figures
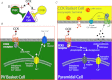
Figure 1. Summarizing intrinsic differences between PV- and CCK-containing basket cells and their synapses
As described in the text, CCK BCs and PV BCs both target the perisomatic region of postsynaptic principal cells, but the distribution of CCK BC synapses is somewhat shifted toward the proximal dendrite, indicating a slight domain specificity. PV BCs (shown in green) have a fast spiking firing pattern (left, green inset) and are primarily activated by feedforward glutamatergic input (blue inputs) with high temporal fidelity while CCK BCs (shown in yellow) have regular spiking firing patterns (right, yellow inset) and because of their intrinsic proclivity for temporal summation are best activated by convergent feedforward and feedback (purple) glutamatergic input. PV BCs and CCK BCs are active at different phases of the theta cycle (upper right grey inset). CCK BCs express M1 (orange oval) and M3 (green oval) muscarinic acetylcholine (mACh) receptors as well as α7 or α4 nicotinic acetylcholine receptors (nACh, maroon pentagon) and 5HT3 receptors (dark blue rectangle). 5HT input (red input) can also affect 5HT1B receptors (pink rectangle) on CA1 pyramidal cell boutons to decrease feedforward excitation of CCK BCs. PV BCs express M1 receptors, extrasynaptic α1-containing GABAA receptors (light blue ovals) and CCK2 receptors (orange lines) coupled to a Gi/o pathway resulting in the opening of TRP channels (dark blue ovals) – non-selective cation channels that depolarize the cell (also see Fig. 2). At the same time, CCK2 receptors on pyramidal cells (orange lines, right circular inset) are coupled to Gq proteins which activate a molecular cascade leading to the synthesis of endocannabinoids (small black octagons). Endocannabinoids synthesized in response to this or other signals travel in a retrograde fashion to the CB1 receptors (green lines) on presynaptic CCK BC terminals to suppress release of GABA (small orange circles). As noted in the text, CCK BCs utilise N-type Ca2+ channels (light blue circles) located at a distance from the GABA release sites, express more GAD65 (grey rounded rectangle) than GAD 67, couple to mainly α2 subunit-containing GABAA receptors (grey oval), and express α oestrogen receptors (brown rectangle) and GABAB receptors (pink star) presynaptically. PV BC synapses (left circular inset) contain abundant GAD65 and GAD67 (tan rounded rectangle) and the GABA transporter GAT1 (blue rounded square), utilise P/Q-type Ca2+ channels (pink circle) located close to GABA release sites, and express μ opioid receptors (yellow oval) and M2 mACh receptors (brown oval), both of which inhibit release. PV BCs couple with mainly α1 subunit-containing GABAA receptors, and their inputs are modulated by the hyperpolarization-activated chloride channel, ClC-2 (green cylinders). Unitary responses in pyramidal cells (purple traces in blue insets, bottom) upon PV BC stimulation (green action potentials) are highly synchronous while responses to CCK BC stimulation (yellow action potentials) exhibit asynchronous release. Electrophysiological traces adapted by permission from Journal of Neuroscience; Lee SY, Földy C, Szabadics J & Soltesz I (2011). Cell-type-specific CCK2 receptor signalling underlies the cholecystokinin-mediated selective excitation of hippocampal parvalbumin-positive fast-spiking basket cells. J Neurosci31,10993–11002, copyright 2011.
Figure 2. Schematic representation of the divergent effects of CCK on PV and CCK basket cells through cell-type-dependent selectivity of CCK2 receptor signalling
A, CCK signalling through the CCK2 receptor exerts opposing effects on PV and CCK basket cells, as described in B and C, with the net effect on PV BCs being depolarization and firing, and the net effect on CCK BCs being a suppression of GABA release. B, in PV BCs, CCK2 receptors couple to an unusual, pertussis toxin-sensitive pathway utilising a Gi/o-coupled mechanism involving cyclic ADP ribose, ryanodine receptors on intracellular calcium stores, and ultimately activating a non-selective cationic conductance through TRP channels. C, in pyramidal cells, CCK2 receptors signal through the more canonical Gq–PLC pathway in which PLCβ produces diacylglycerol (DAG) which is converted by DAG lipase to endocannabinoids that can travel in a retrograde fashion to cannabinoid type 1 (CB1) receptors on presynaptic CCK basket cell terminals, decreasing GABA release. Adapted by permission from Journal of Neuroscience; Lee SY, Földy C, Szabadics J & Soltesz I (2011). Cell-type-specific CCK2 receptor signalling underlies the cholecystokinin-mediated selective excitation of hippocampal parvalbumin-positive fast-spiking basket cells. J Neurosci31,10993–11002, copyright 2011.
Figure 3. Subnetwork-specific targeting by CCK BCs in the medial entorhinal cortex layer II (MEClayerII)
A, in medial entorhinal cortex layer II (MEClayerII), CCK-positive punctae (yellow) surround only certain principal cells (labelled using GluR2/3, purple, targeted cells noted by stars) and avoid others (noted by triangles) while PV punctae (green) do not appear selective. B, the cells targeted by CCK BC terminals (labelled using a marker of CCK BC boutons, VGLUT3, yellow) selectively innervate the calbindin-containing principal cells (red) that project to the contralateral entorhinal cortex (EC), avoiding reelin-containing principal cells (blue) that form the perforant path to the ipsilateral dentate gyrus (DG). C, summary diagram representing the arrangement of perisomatic boutons in MEClayerII. Perisomatic PV-containing punctae (green) are seen surrounding both principal cell populations while CCK BCs select only calbindin-containing principal cells. Parts A and B adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience Varga C, Lee SY & Soltesz I (2010). Target-selective GABAergic control of entorhinal cortex output. Nat Neurosci13, 822–824, copyright 2010.
Similar articles
- Differential surface density and modulatory effects of presynaptic GABAB receptors in hippocampal cholecystokinin and parvalbumin basket cells.
Booker SA, Althof D, Degro CE, Watanabe M, Kulik Á, Vida I. Booker SA, et al. Brain Struct Funct. 2017 Nov;222(8):3677-3690. doi: 10.1007/s00429-017-1427-x. Epub 2017 May 2. Brain Struct Funct. 2017. PMID: 28466358 Free PMC article. - Properties and dynamics of inhibitory synaptic communication within the CA3 microcircuits of pyramidal cells and interneurons expressing parvalbumin or cholecystokinin.
Kohus Z, Káli S, Rovira-Esteban L, Schlingloff D, Papp O, Freund TF, Hájos N, Gulyás AI. Kohus Z, et al. J Physiol. 2016 Jul 1;594(13):3745-74. doi: 10.1113/JP272231. Epub 2016 May 5. J Physiol. 2016. PMID: 27038232 Free PMC article. - Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells.
Bartos M, Elgueta C. Bartos M, et al. J Physiol. 2012 Feb 15;590(4):669-81. doi: 10.1113/jphysiol.2011.226175. Epub 2012 Jan 16. J Physiol. 2012. PMID: 22250212 Free PMC article. Review. - Cortical basket cell dysfunction in schizophrenia.
Curley AA, Lewis DA. Curley AA, et al. J Physiol. 2012 Feb 15;590(4):715-24. doi: 10.1113/jphysiol.2011.224659. Epub 2012 Jan 4. J Physiol. 2012. PMID: 22219337 Free PMC article. Review.
Cited by
- Traumatic Brain Injury Diminishes Feedforward Activation of Parvalbumin-Expressing Interneurons in the Dentate Gyrus.
Folweiler KA, Xiong G, Best KM, Metheny HE, Nah G, Cohen AS. Folweiler KA, et al. eNeuro. 2020 Nov 13;7(6):ENEURO.0195-19.2020. doi: 10.1523/ENEURO.0195-19.2020. Print 2020 Nov/Dec. eNeuro. 2020. PMID: 33106385 Free PMC article. - Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive.
Rosen ZB, Cheung S, Siegelbaum SA. Rosen ZB, et al. Nat Neurosci. 2015 Dec;18(12):1763-71. doi: 10.1038/nn.4152. Epub 2015 Nov 2. Nat Neurosci. 2015. PMID: 26523642 Free PMC article. - Navigating the circuitry of the brain's GPS system: Future challenges for neurophysiologists.
Craig MT, McBain CJ. Craig MT, et al. Hippocampus. 2015 Jun;25(6):736-43. doi: 10.1002/hipo.22456. Epub 2015 Apr 2. Hippocampus. 2015. PMID: 25786788 Free PMC article. - On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy.
Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. Krook-Magnuson E, et al. Nat Commun. 2013;4:1376. doi: 10.1038/ncomms2376. Nat Commun. 2013. PMID: 23340416 Free PMC article. - Ventral hippocampal cholecystokinin interneurons gate contextual reward memory.
Nguyen R, Sivakumaran S, Lambe EK, Kim JC. Nguyen R, et al. iScience. 2024 Jan 9;27(2):108824. doi: 10.1016/j.isci.2024.108824. eCollection 2024 Feb 16. iScience. 2024. PMID: 38303709 Free PMC article.
References
- Ali AB, Todorova M. Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. Eur J Neurosci. 2010;31:1196–1207. - PubMed
- Baimbridge KG, Peet MJ, McLennan H, Church J. Bursting response to current-evoked depolarization in rat CA1 pyramidal neurons is correlated with lucifer yellow dye coupling but not with the presence of calbindin-D28k. Synapse. 1991;7:269–277. - PubMed
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS035915/NS/NINDS NIH HHS/United States
- R37 NS035915/NS/NINDS NIH HHS/United States
- NS35915/NS/NINDS NIH HHS/United States
- R29 NS035915/NS/NINDS NIH HHS/United States
- R01 NS074432/NS/NINDS NIH HHS/United States
- NS74432/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources