Regulation of glucose and glycogen metabolism during and after exercise - PubMed (original) (raw)
Review
Regulation of glucose and glycogen metabolism during and after exercise
Thomas E Jensen et al. J Physiol. 2012.
Abstract
Utilization of carbohydrate in the form of intramuscular glycogen stores and glucose delivered from plasma becomes an increasingly important energy substrate to the working muscle with increasing exercise intensity. This review gives an update on the molecular signals by which glucose transport is increased in the contracting muscle followed by a discussion of glycogen mobilization and synthesis by the action of glycogen phosphorylase and glycogen synthase, respectively. Finally, this review deals with the signalling relaying the well-described increased sensitivity of glucose transport to insulin in the post-exercise period which can result in an overshoot of intramuscular glycogen resynthesis post exercise (glycogen supercompensation).
Figures
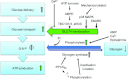
Figure 1. Glucose utilization in the working muscle is increased through increased delivery and uptake of plasma glucose and increased glycogenolysis
Transport of glucose across the sarcolemma and T-tubular membranes is determined by the amount of contraction- and insulin-responsive glucose transporter 4 (GLUT4) proteins in the outer membrane. This magnitude of glucose transport response with contraction correlates with work intensity with evidence suggesting the involvement of kinases like AMPK, p38 MAPK and SNARK whereas Ca2+ activated proteins are probably required but likely to be insufficient to stimulate glucose transport. Allosteric and covalent regulation increases both glycogen mobilization by glycogen phosphorylase (GP) and resynthesis by glycogen synthase (GS) simultaneously during exercise by altering enzyme activity and/or location. GP may also be regulated by the availability of its substrates glycogen and inorganic phosphate (Pi). Depending on the work intensity and duration, glucose-6-phosphate (G-6-P), an important allosteric inhibitor of GP and stimulator of GS, may increase.
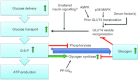
Figure 2. Augmented glycogen resynthesis post-exercise is explained to a large part by sensitization of the insulin-stimulated glucose transport response and glycogen synthase activation
Although some signalling proteins display a prolonged increase in phosphorylation for many hours after exercise, perhaps contributing to insulin sensitization, many insulin-signalling endpoints seem unaffected by prior contraction. Mobilization of GLUT4 during contraction may cause a subsequent sorting into a more insulin-responsive pool or position. Insulin sensitization may require permissive input from one or more unidentified serum factors. Increased amounts of transported glucose are converted to glucose-6-phosphate (G-6-P) which allosterically increases glycogen synthase and inhibits phosphorylase activity, respectively. High glycogen shows a correlation with decreased insulin-stimulated glucose transport and glycogen synthase inactivation but whether this is a causal relationship remains unclear.
Similar articles
- Exercise-induced molecular mechanisms promoting glycogen supercompensation in human skeletal muscle.
Hingst JR, Bruhn L, Hansen MB, Rosschou MF, Birk JB, Fentz J, Foretz M, Viollet B, Sakamoto K, Færgeman NJ, Havelund JF, Parker BL, James DE, Kiens B, Richter EA, Jensen J, Wojtaszewski JFP. Hingst JR, et al. Mol Metab. 2018 Oct;16:24-34. doi: 10.1016/j.molmet.2018.07.001. Epub 2018 Jul 25. Mol Metab. 2018. PMID: 30093357 Free PMC article. - Muscle glycogen and metabolic regulation.
Hargreaves M. Hargreaves M. Proc Nutr Soc. 2004 May;63(2):217-20. doi: 10.1079/PNS2004344. Proc Nutr Soc. 2004. PMID: 15294033 Review. - The putative roles of adenosine in insulin- and exercise-mediated regulation of glucose transport and glycogen metabolism in skeletal muscle.
Thong FS, Graham TE. Thong FS, et al. Can J Appl Physiol. 2002 Apr;27(2):152-78. doi: 10.1139/h02-011. Can J Appl Physiol. 2002. PMID: 12184210 Review. - Regulation of glycogen resynthesis following exercise. Dietary considerations.
Friedman JE, Neufer PD, Dohm GL. Friedman JE, et al. Sports Med. 1991 Apr;11(4):232-43. doi: 10.2165/00007256-199111040-00003. Sports Med. 1991. PMID: 1901662 Review. - 1997 Sir William Refshauge Lecture. Skeletal muscle glucose metabolism during exercise: implications for health and performance.
Hargreaves M. Hargreaves M. J Sci Med Sport. 1998 Dec;1(4):195-202. doi: 10.1016/s1440-2440(09)60001-3. J Sci Med Sport. 1998. PMID: 9923727 Review.
Cited by
- Energy Metabolism in Exercise-Induced Physiologic Cardiac Hypertrophy.
Xiang K, Qin Z, Zhang H, Liu X. Xiang K, et al. Front Pharmacol. 2020 Jul 29;11:1133. doi: 10.3389/fphar.2020.01133. eCollection 2020. Front Pharmacol. 2020. PMID: 32848751 Free PMC article. Review. - Fluoride as a Potential Repressor of Glycogen Metabolism in Skeletal Muscle Cell Line CCL136.
Gutowska I, Maruszewska A, Skórka-Majewicz M, Kempińska-Podhorodecka A, Kolasa A, Wszołek A, Baranowska-Bosiacka I, Żwierełło W. Gutowska I, et al. Molecules. 2023 Aug 15;28(16):6065. doi: 10.3390/molecules28166065. Molecules. 2023. PMID: 37630316 Free PMC article. - Concentric and Eccentric Endurance Exercise Reverse Hallmarks of T-Cell Senescence in Pre-diabetic Subjects.
Philippe M, Gatterer H, Burtscher M, Weinberger B, Keller M, Grubeck-Loebenstein B, Fleckenstein J, Alack K, Krüger K. Philippe M, et al. Front Physiol. 2019 Jun 4;10:684. doi: 10.3389/fphys.2019.00684. eCollection 2019. Front Physiol. 2019. PMID: 31214051 Free PMC article. - The causal pathway effects of a physical activity intervention on adiposity in children: The KISS Study cluster randomized clinical trial.
Lima RA, Andersen LB, Soares FC, Kriemler S. Lima RA, et al. Scand J Med Sci Sports. 2020 Sep;30(9):1685-1691. doi: 10.1111/sms.13741. Epub 2020 Jun 22. Scand J Med Sci Sports. 2020. PMID: 32501613 Free PMC article. Clinical Trial. - A Novel Phenylchromane Derivative Increases the Rate of Glucose Uptake in L6 Myotubes and Augments Insulin Secretion from Pancreatic Beta-Cells by Activating AMPK.
Rozentul N, Avrahami Y, Shubely M, Levy L, Munder A, Cohen G, Cerasi E, Sasson S, Gruzman A. Rozentul N, et al. Pharm Res. 2017 Dec;34(12):2873-2890. doi: 10.1007/s11095-017-2271-7. Epub 2017 Oct 5. Pharm Res. 2017. PMID: 28983714
References
- Antonescu CN, Huang C, Niu W, Liu Z, Eyers PA, Heidenreich KA, Bilan PJ, Klip A. Reduction of insulin-stimulated glucose uptake in L6 myotubes by the protein kinase inhibitor SB203580 is independent of p38MAPK activity. Endocrinology. 2005;146:3773–3781. - PubMed
- Aschenbach WG, Suzuki Y, Breeden K, Prats C, Hirshman MF, Dufresne SD, Sakamoto K, Vilardo PG, Steele M, Kim JH, Jing SL, Goodyear LJ, DePaoli-Roach AA. The muscle-specific protein phosphatase PP1G/R(GL)(G(M))is essential for activation of glycogen synthase by exercise. J Biol Chem. 2001;276:39959–39967. - PubMed
- Bouskila M, Hunter RW, Ibrahim AF, Delattre L, Peggie M, Diepen vanJA, Voshol PJ, Jensen J, Sakamoto K. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle. Cell Metab. 2010;12:456–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical