From the regulation of peptidoglycan synthesis to bacterial growth and morphology - PubMed (original) (raw)
Review
From the regulation of peptidoglycan synthesis to bacterial growth and morphology
Athanasios Typas et al. Nat Rev Microbiol. 2011.
Abstract
How bacteria grow and divide while retaining a defined shape is a fundamental question in microbiology, but technological advances are now driving a new understanding of how the shape-maintaining bacterial peptidoglycan sacculus grows. In this Review, we highlight the relationship between peptidoglycan synthesis complexes and cytoskeletal elements, as well as recent evidence that peptidoglycan growth is regulated from outside the sacculus in Gram-negative bacteria. We also discuss how growth of the sacculus is sensitive to mechanical force and nutritional status, and describe the roles of peptidoglycan hydrolases in generating cell shape and of D-amino acids in sacculus remodelling.
Conflict of interest statement
Competing interests statement
The authors declare no competing financial interests.
Figures
Figure 1. Peptidoglycan synthesis and cleavage
The synthesis and attachment of a new peptidoglycan strand to the existing sacculus, with particular emphasis on the different synthetic and degrading enzymes. Precursors are synthesized in the cytoplasm, linked to the transport lipid (undecaprenyl phosphate) and flipped accross the inner membrane by FtsW–RodA. A glycosyltransferase (GTase) catalyses polymerization of a nascent peptidoglycan chain from lipid II precursor at the inner membrane, followed by attachment of the new chain to the sacculus by a DD-transpeptidase (DD-TPase). Peptides are trimmed by DD-, LD-and DL-carboxypeptidases (CPases), and crosslinks are cleaved by the DD-and LD-endopeptidases (EPases). Amidases remove peptides from glycan chains, and _exo_-or endo-specific lytic transglycosylases (LTs) cleave in the glycan chain to form 1,6-anhydro-_N_-acetylmuramic acid (anhMurNAc) residues, which are the hallmark of glycan chain ends. LD-TPases are responsible for the formation of LD-crosslinks, the attachment of the major outer-membrane lipoprotein (Lpp), which is anchored in the outer membrane, and the binding of unusual D-amino acids. The number of known Escherichia coli enzymes for each group is shown in brackets, but this is probably an underestimate, as even in E. coli not all players are known and/or characterized. Alr, Ala racemase, biosynthetic; DadX, Ala racemase, catabolic; DdlA, D-Ala–D-Ala ligase A; GlcNAc, _N_-acetylglucosamine; _meso_-Dap, _meso_-diaminopimelic acid; MraY, UDP-MurNAc-pentapeptide phosphotransferase; MurA, UDP-GlcNAc enolpyruvyl transferase; MurB, UDP-MurNAc dehydrogenase; MurC, UDP-MurNAc–L-Ala ligase; MurD, UDP-MurNAc-L-Ala–D-Glu ligase; MurE, UDP-MurNAc-L-Ala-D-Glu-_meso_-Dap ligase; MurF, UDP-MurNAc-tripeptide–D-alanyl-D-Ala ligase; MurG, UDP-GlcNAc-undecaprenoyl-pyrophosphoryl-MurNAc-pentapeptide transferase; Murl, Glu racemase; PEP, phosphoenolpyruvate.
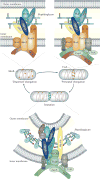
Figure 2. Different peptidoglycan synthesis complexes are active at different stages of the Escherichia coli cell cycle
As shown in the upper left panel, MreB and associated membrane proteins control or position the peptidoglycan synthases penicillin-binding protein 1A (PBP1A) and PBP2, as well as still-unknown hydrolases (Hyd), during the ‘dispersed’ mode of elongation. As illustrated in the upper right panel, FtsZ and other early cell division proteins control the elongation-specific peptidoglycan synthesis complex during a ‘preseptal’ mode of elongation. It is not known whether MreB and associated proteins participate in preseptal elongation. Finally, as depicted in the lower panel, the cell division complex contains essential, inner membrane-localized cell division proteins, the peptidoglycan synthases PBP1B and PBP3, and amidase enzymes (Ami) with their activators, as well as proteins of the Tol–Pal complex for constriction of the outer membrane. Activity of the PBPs is regulated in part by outer membrane-anchored lipoproteins such as LpoA and LpoB. LT, lytic transglycosylase.
Figure 3. Force generation by cytoskeletal elements
a | Crescentin (CreS) reduces the strain at one side of the cell, causing Caulobacter crescentusto grow in a bent shape. Detachment of the CreS filament from the membrane (on addition of mecillinam) results in rapid loss of the filament’s stretched form but does not cause an instant change in cell shape. Cells lacking CreS grow with a straight shape. b | FtsZ generates an inwardly directed constriction force in vesicle tubes and presumably also in the cell. c | Depolymerization of MreB filaments by addition of the drug A22 (_S_-(3,4-dichlorobenzyl)isothiourea) reduces the stiffness of Escherichia coli cells.
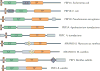
Figure 4. Species-specific non-catalytic regions in penicillin-binding proteins
Different class A penicillin-binding proteins (PBPs) in comparison with Escherichia coli PBP1A and PBP1B. Predicted or known transmembrane domains are shown in brown, newly evolved domains in E. coli PBP1A and PBP1B in dark blue and other species-specific regions with no function prediction in grey. Glycosyltransferase (GT) and transpeptidase (TP) domains are labelled, along with the fibronectin type 3 (FN3) domain and the ribosomal protein S1-like RNA-binding (S1) domains. The species-specific regions with no function prediction in the two Myxococcus xanthus proteins contain an S1 domain and are only conserved in Stigmatella and Myxococcus spp., whereas the analogous regions in the two Bacillus subtilis proteins consist of one that is unique in B. subtilis (the carboxy-terminal region in PBP1) and one that is more conserved among the bacilli (the domain in PBP4); the FN3 domain found in PBP1 is also conserved only in bacilli. ODD, outer-membrane PBP1A docking domain; UB2H, UvrB domain 2 homologue.
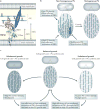
Figure 5. Regulation of peptidoglycan synthesis by outer-membrane proteins
a | Side view of the Escherichia coli cell envelope with the crystal structure of penicillin-binding protein 1B (PBP1B; Protein Data Bank accession 3FWM) and the distances between the inner membrane, peptidoglycan (PG) and outer membrane drawn to scale. The glycosyltransferase (GTase) and transpeptidase (TPase) domains are shown. The structure of the activator protein for PBP1B, LpoB, is unknown. LpoB is anchored in the outer membrane and interacts with the PBP1B UB2H (UvrB domain 2 homologue) domain, which is situated between the inner membrane and the PG layer, not more than ~60 Å away from the inner membrane. The distance from the inner membrane to the PG is ~90 Å. b | A hypothetical self-repair mechanism to maintain a homogeneous peptidoglycan layer. The cell on the left has a non-homogeneous peptidoglycan layer consisting of large and small pores. Pore size-responsive activation of peptidoglycan synthase activity results in a more homogeneous peptidoglycan layer (on the right). c | A hypothetical homeostatic mechanism to balance the peptidoglycan growth rate with the overall cellular growth rate. When the peptidoglycan growth rate falls behind or exceeds that of overall cell growth, the peptidoglycan net stretches or relaxes, respectively. The resulting change in pore size alters the efficiency with which Lpo proteins can activate peptidoglycan synthases and therefore re-aligns the peptidoglycan growth rate with the overall cellular growth rate.
Comment in
- Bacterial physiology: Flipping out over MurJ.
Kåhrström CT. Kåhrström CT. Nat Rev Microbiol. 2014 Sep;12(9):595. doi: 10.1038/nrmicro3328. Epub 2014 Jul 21. Nat Rev Microbiol. 2014. PMID: 25043163 No abstract available.
Similar articles
- Outer membrane lipoprotein NlpI scaffolds peptidoglycan hydrolases within multi-enzyme complexes in Escherichia coli.
Banzhaf M, Yau HC, Verheul J, Lodge A, Kritikos G, Mateus A, Cordier B, Hov AK, Stein F, Wartel M, Pazos M, Solovyova AS, Breukink E, van Teeffelen S, Savitski MM, den Blaauwen T, Typas A, Vollmer W. Banzhaf M, et al. EMBO J. 2020 Mar 2;39(5):e102246. doi: 10.15252/embj.2019102246. Epub 2020 Feb 3. EMBO J. 2020. PMID: 32009249 Free PMC article. - ZipA is required for FtsZ-dependent preseptal peptidoglycan synthesis prior to invagination during cell division.
Potluri LP, Kannan S, Young KD. Potluri LP, et al. J Bacteriol. 2012 Oct;194(19):5334-42. doi: 10.1128/JB.00859-12. Epub 2012 Jul 27. J Bacteriol. 2012. PMID: 22843850 Free PMC article. - The active repertoire of Escherichia coli peptidoglycan amidases varies with physiochemical environment.
Mueller EA, Iken AG, Ali Öztürk M, Winkle M, Schmitz M, Vollmer W, Di Ventura B, Levin PA. Mueller EA, et al. Mol Microbiol. 2021 Jul;116(1):311-328. doi: 10.1111/mmi.14711. Epub 2021 Apr 3. Mol Microbiol. 2021. PMID: 33666292 Free PMC article. - Regulation of peptidoglycan synthesis and remodelling.
Egan AJF, Errington J, Vollmer W. Egan AJF, et al. Nat Rev Microbiol. 2020 Aug;18(8):446-460. doi: 10.1038/s41579-020-0366-3. Epub 2020 May 18. Nat Rev Microbiol. 2020. PMID: 32424210 Review. - Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli.
Vollmer W, Bertsche U. Vollmer W, et al. Biochim Biophys Acta. 2008 Sep;1778(9):1714-34. doi: 10.1016/j.bbamem.2007.06.007. Epub 2007 Jun 16. Biochim Biophys Acta. 2008. PMID: 17658458 Review.
Cited by
- Precise regulation of the relative rates of surface area and volume synthesis in bacterial cells growing in dynamic environments.
Shi H, Hu Y, Odermatt PD, Gonzalez CG, Zhang L, Elias JE, Chang F, Huang KC. Shi H, et al. Nat Commun. 2021 Mar 30;12(1):1975. doi: 10.1038/s41467-021-22092-5. Nat Commun. 2021. PMID: 33785742 Free PMC article. - Phenotypic analysis of Eschericia coli mutants lacking L,D-transpeptidases.
Sanders AN, Pavelka MS. Sanders AN, et al. Microbiology (Reading). 2013 Sep;159(Pt 9):1842-1852. doi: 10.1099/mic.0.069211-0. Epub 2013 Jul 7. Microbiology (Reading). 2013. PMID: 23832002 Free PMC article. - Symmetry and scale orient Min protein patterns in shaped bacterial sculptures.
Wu F, van Schie BG, Keymer JE, Dekker C. Wu F, et al. Nat Nanotechnol. 2015 Aug;10(8):719-26. doi: 10.1038/nnano.2015.126. Epub 2015 Jun 22. Nat Nanotechnol. 2015. PMID: 26098227 Free PMC article. - Peptidoglycan fragment release from Neisseria meningitidis.
Woodhams KL, Chan JM, Lenz JD, Hackett KT, Dillard JP. Woodhams KL, et al. Infect Immun. 2013 Sep;81(9):3490-8. doi: 10.1128/IAI.00279-13. Epub 2013 Jul 8. Infect Immun. 2013. PMID: 23836824 Free PMC article. - Molecular mechanisms for the evolution of bacterial morphologies and growth modes.
Randich AM, Brun YV. Randich AM, et al. Front Microbiol. 2015 Jun 9;6:580. doi: 10.3389/fmicb.2015.00580. eCollection 2015. Front Microbiol. 2015. PMID: 26106381 Free PMC article. Review.
References
- Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32:149–167. - PubMed
- Barreteau H, et al. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:168–207. - PubMed
- Bouhss A, Trunkfield AE, Bugg TD, Mengin-Lecreulx D. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol Rev. 2008;32:208–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM085697/GM/NIGMS NIH HHS/United States
- GM085697/GM/NIGMS NIH HHS/United States
- GM085697‑01S1/GM/NIGMS NIH HHS/United States
- R01 GM036278/GM/NIGMS NIH HHS/United States
- K99GM092984/GM/NIGMS NIH HHS/United States
- BB/G015902/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBI020012/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- K99 GM092984/GM/NIGMS NIH HHS/United States
- BB/I020012/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases