Mitochondrial dysregulation in the pathogenesis of diabetes: potential for mitochondrial biogenesis-mediated interventions - PubMed (original) (raw)
Review
Mitochondrial dysregulation in the pathogenesis of diabetes: potential for mitochondrial biogenesis-mediated interventions
Anna-Maria Joseph et al. Exp Diabetes Res. 2012.
Abstract
Muscle mitochondrial metabolism is a tightly controlled process that involves the coordination of signaling pathways and factors from both the nuclear and mitochondrial genomes. Perhaps the most important pathway regulating metabolism in muscle is mitochondrial biogenesis. In response to physiological stimuli such as exercise, retrograde signaling pathways are activated that allow crosstalk between the nucleus and mitochondria, upregulating hundreds of genes and leading to higher mitochondrial content and increased oxidation of substrates. With type 2 diabetes, these processes can become dysregulated and the ability of the cell to respond to nutrient and energy fluctuations is diminished. This, coupled with reduced mitochondrial content and altered mitochondrial morphology, has been directly linked to the pathogenesis of this disease. In this paper, we will discuss our current understanding of mitochondrial dysregulation in skeletal muscle as it relates to type 2 diabetes, placing particular emphasis on the pathways of mitochondrial biogenesis and mitochondrial dynamics, and the therapeutic value of exercise and other interventions.
Figures
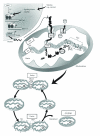
Figure 1
Proposed model of mitochondrial biogenesis. In response to a stimulus such as skeletal muscle contractile activity or exercise, intracellular Ca2+ levels, as well as AMP levels, increase leading to the activation of signaling molecules including AMP-activated protein kinase (AMPK). These signaling pathways converge and interact primarily with the transcriptional coactivator peroxisome proliferator-activated receptor-γ coactivator-1_α_ (PGC-1_α_) which is a master regulator of mitochondrial biogenesis. PGC-1_α_ activates its own expression, as well as the expression of the nuclear respiratory factor-1 and 2 (NRF-1/2). Additionally, PGC-1_α_ has recently been shown to be deacetylated and activated by the longevity protein sirtuin 1 (SIRT1). NRF-1 and NRF-2 bind and upregulate the expression of nuclear genes encoding mitochondrial proteins (NUGEMPs), as well as the expression of mitochondrial transcription factor A (Tfam). Tfam along with other newly transcribed NUGEMPS are targeted and imported into mitochondrial subcompartments via the protein import machinery (PIM). Within the matrix, Tfam binds to mtDNA and regulates the expression of the 13 mitochondrial DNA (mtDNA) gene products. These proteins are assembled into multisubunit enzyme complexes within the electron transport chain (ETC) and mediate oxidative phosphorylation (OXPHOS) and the production of ATP. Thus, coordinated expression regulated by the two genomes allows for the proper assembly and expansion of the mitochondrial reticulum leading to mitochondrial proliferation and increased mitochondrial number/content. Another important product of the ETC is reactive oxygen species (ROS) that are associated with the mitochondrial membrane potential (Δ_ψ_ m). Elevated levels of ROS have been shown to activate mitochondrial outer membrane permeabilization (MOMP) and the release of proapoptotic factors such as cytochrome c (Cyt c) into the cytosol that can subsequently activate caspase-dependent signaling cascades leading to mitochondrially-mediated apoptosis. Furthermore, organelle biogenesis requires a continuous cycle of fusion and fission events. Mitochondrial fusion of the outer and inner mitochondrial membranes is mediated by the GTPase proteins, mitofusin 1 and 2 (Mfn1 and Mfn2) and OPA1, respectively. Conversely, mitochondrial fission requires Drp1 and Fis1 which assemble at fission sites on the mitochondrial membrane and induce membrane division. It has been proposed that fission can lead to mitochondria with different Δ_ψ_ m and that damaged or depolarized organelles will exit the fusion and fission cycle and will be removed through autophagy.
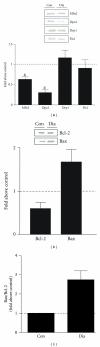
Figure 2
(a) Mitochondrial morphology proteins in type 2 diabetes. The research volunteers that participated in this study were obese subjects with type 2 diabetes undergoing coronary bypass surgery and were all male between 48 and 75 years of age. Biopsies from the vastus medialis muscle were removed from both control and type 2 diabetic subjects from within incisions of the inner thigh and protein analyses performed. The protocol was approved by the Medical Ethics Committees of Laval University and Laval Hospital, and all subjects provided informed written consent. Representative western blots of fusion proteins Mfn2 and OPA1 and fission proteins Drp1 and Fis1 from the vastus medialis muscle of control (Con) and type 2 diabetic subjects (Dia). A summary of repeated experiments is shown below with values expressed as a fold over control. Values are means ± SE; n = 4–9; *P < 0.05 versus Con. (b) Indicators of apoptotic susceptibility in type 2 diabetes. Western blots of the antiapoptotic protein Bcl-2 and the proapoptotic protein Bax in vastus medialis muscle of Con and Dia individuals and the graphical representation of the data is shown below. Values are means ± SE; n = 4–9. (c) The ratio of Bax/Bcl-2 in Dia subjects when compared to Con.
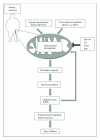
Figure 3
Simplified scheme that illustrates the role of mitochondrial dysregulation in the pathogenesis of type 2 diabetes in skeletal muscle. Obesity and physical inactivity can result in mitochondrial dysregulation through alterations in crucial transcriptional activators (e.g., PGC-1_α_ and SIRT1), as well as impaired fusion and fission leading to aberrant mitochondrial morphology. These changes can subsequently lead to reduced oxidative capacity and cause lipid metabolite accumulation, increased oxidative stress, and the production of reactive oxygen species (ROS). Over time, the accumulation of ROS can damage DNA, proteins, and lipids, further exacerbating mitochondrial dysfunction. Collectively, these factors contribute to impaired insulin signaling pathways and increase the risk of type 2 diabetes. On the other hand, physiological interventions, including exercise and caloric restriction (CR), as well as pharmacological agents such as thiazolidinediones (TZDs) and resveratrol (RSV) have been shown to stimulate mitochondrial biogenesis and reduce mitochondrial dysfunction that is observed with type 2 diabetes in muscle.
Similar articles
- Skeletal muscle mitochondrial remodeling in exercise and diseases.
Gan Z, Fu T, Kelly DP, Vega RB. Gan Z, et al. Cell Res. 2018 Oct;28(10):969-980. doi: 10.1038/s41422-018-0078-7. Epub 2018 Aug 14. Cell Res. 2018. PMID: 30108290 Free PMC article. Review. - Insulin Resistance and Mitochondrial Dysfunction.
Gonzalez-Franquesa A, Patti ME. Gonzalez-Franquesa A, et al. Adv Exp Med Biol. 2017;982:465-520. doi: 10.1007/978-3-319-55330-6_25. Adv Exp Med Biol. 2017. PMID: 28551803 Review. - Skeletal Muscle Nucleo-Mitochondrial Crosstalk in Obesity and Type 2 Diabetes.
Devarshi PP, McNabney SM, Henagan TM. Devarshi PP, et al. Int J Mol Sci. 2017 Apr 14;18(4):831. doi: 10.3390/ijms18040831. Int J Mol Sci. 2017. PMID: 28420087 Free PMC article. Review. - Pioglitazone treatment restores in vivo muscle oxidative capacity in a rat model of diabetes.
Wessels B, Ciapaite J, van den Broek NM, Houten SM, Nicolay K, Prompers JJ. Wessels B, et al. Diabetes Obes Metab. 2015 Jan;17(1):52-60. doi: 10.1111/dom.12388. Epub 2014 Oct 6. Diabetes Obes Metab. 2015. PMID: 25200673 - The role of AMPK in controlling metabolism and mitochondrial biogenesis during exercise.
Marcinko K, Steinberg GR. Marcinko K, et al. Exp Physiol. 2014 Dec 1;99(12):1581-5. doi: 10.1113/expphysiol.2014.082255. Epub 2014 Sep 25. Exp Physiol. 2014. PMID: 25261498 Review.
Cited by
- Complex I inhibition in the visual pathway induces disorganization of the node of Ranvier.
Marella M, Patki G, Matsuno-Yagi A, Yagi T. Marella M, et al. Neurobiol Dis. 2013 Oct;58:281-8. doi: 10.1016/j.nbd.2013.06.010. Epub 2013 Jun 29. Neurobiol Dis. 2013. PMID: 23816754 Free PMC article. - Differential remodelling of mitochondrial subpopulations and mitochondrial dysfunction are a feature of early stage diabetes.
Rajab BS, Kassab S, Stonall CD, Daghistani H, Gibbons S, Mamas M, Smith D, Mironov A, AlBalawi Z, Zhang YH, Baudoin F, Zi M, Prehar S, Cartwright EJ, Kitmitto A. Rajab BS, et al. Sci Rep. 2022 Jan 19;12(1):978. doi: 10.1038/s41598-022-04929-1. Sci Rep. 2022. PMID: 35046471 Free PMC article. - Head to Head Comparison of Short-Term Treatment with the NAD(+) Precursor Nicotinamide Mononucleotide (NMN) and 6 Weeks of Exercise in Obese Female Mice.
Uddin GM, Youngson NA, Sinclair DA, Morris MJ. Uddin GM, et al. Front Pharmacol. 2016 Aug 19;7:258. doi: 10.3389/fphar.2016.00258. eCollection 2016. Front Pharmacol. 2016. PMID: 27594836 Free PMC article. - Activity-induced changes in skeletal muscle metabolism measured with optical spectroscopy.
Ryan TE, Southern WM, Brizendine JT, McCully KK. Ryan TE, et al. Med Sci Sports Exerc. 2013 Dec;45(12):2346-52. doi: 10.1249/MSS.0b013e31829a726a. Med Sci Sports Exerc. 2013. PMID: 23669881 Free PMC article. - Post-translational modifications and protein quality control of mitochondrial channels and transporters.
Kadam A, Jadiya P, Tomar D. Kadam A, et al. Front Cell Dev Biol. 2023 Aug 3;11:1196466. doi: 10.3389/fcell.2023.1196466. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37601094 Free PMC article. Review.
References
- Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40(11):1397–1403. - PubMed
- Russell JC, Shillabeer G, Bar-Tana J, et al. Development of insulin resistance in the JCR:LA-cp rat: role of triacylglycerols and effects of MEDICA 16. Diabetes. 1998;47(5):770–778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical